Folic acid functionalization for targeting self-assembled paclitaxel-based nanoparticles
- PMID: 36544466
- PMCID: PMC9744106
- DOI: 10.1039/d2ra06306a
Folic acid functionalization for targeting self-assembled paclitaxel-based nanoparticles
Abstract
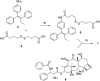
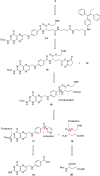
Hetero-nanoparticles self-assembled from a conjugate bearing folic acid as the targeting agent, and another bearing paclitaxel as the active agent are reported. Hetero-nanoparticles containing varying percentages of folic acid conjugates are characterised, and their biological activity is determined.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


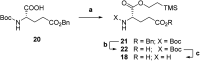





References
-
- Wang Y. Santos A. Evdokiou A. Losic D. J. Mater. Chem. B. 2015;3:7153–7172. - PubMed
-
- Zhang Y. Fang F. Li L. Zhang J. ACS Biomater. Sci. Eng. 2020;6:4816–4833. - PubMed
-
- Borrelli S. Christodoulou M. S. Ficarra I. Silvani A. Cappelletti G. Cartelli D. Damia G. Ricci F. Zucchetti M. Dosio F. Passarella D. Eur. J. Med. Chem. 2014;85:179–190. - PubMed

