The three-center two-positron bond
- PMID: 36544737
- PMCID: PMC9710307
- DOI: 10.1039/d2sc04630j
The three-center two-positron bond
Abstract
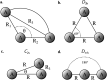
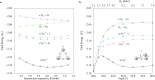
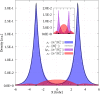
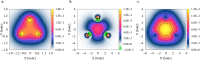
Computational studies have shown that one or more positrons can stabilize two repelling atomic anions through the formation of two-center positronic bonds. In the present work, we study the energetic stability of a system containing two positrons and three hydride anions, namely 2e+[H3 3-]. To this aim, we performed a preliminary scan of the potential energy surface of the system with both electrons and positrons in a spin singlet state, with a multi-component MP2 method, that was further refined with variational and diffusion Monte Carlo calculations, and confirmed an equilibrium geometry with D 3h symmetry. The local stability of 2e+[H3 3-] is demonstrated by analyzing the vertical detachment and adiabatic energy dissociation channels. Bonding properties of the positronic compound, such as the equilibrium interatomic distances, force constants, dissociation energies, and bonding densities are compared with those of the purely electronic H3 + and Li3 + systems. Through this analysis, we find compelling similarities between the 2e+[H3 3-] compound and the trilithium cation. Our results strongly point out the formation of a non-electronic three-center two-positron bond, analogous to the well-known three-center two-electron counterparts, which is fundamentally distinct from the two-center two-positron bond [D. Bressanini, J. Chem. Phys., 2021, 155, 054306], thus extending the concept of positron bonded molecules.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
LinkOut - more resources
Full Text Sources

