Uranium(iv) alkyl cations: synthesis, structures, comparison with thorium(iv) analogues, and the influence of arene-coordination on thermal stability and ethylene polymerization activity
- PMID: 36544741
- PMCID: PMC9710223
- DOI: 10.1039/d2sc04302e
Uranium(iv) alkyl cations: synthesis, structures, comparison with thorium(iv) analogues, and the influence of arene-coordination on thermal stability and ethylene polymerization activity
Abstract
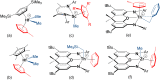
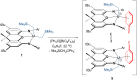
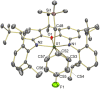
Reaction of [(XA2)U(CH2SiMe3)2] (1; XA2 = 4,5-bis(2,6-diisopropylanilido)-2,7-di-tert-butyl-9,9-dimethylxanthene) with 1 equivalent of [Ph3C][B(C6F5)4] in arene solvents afforded the arene-coordinated uranium alkyl cations, [(XA2)U(CH2SiMe3)(η n -arene)][B(C6F5)4] {arene = benzene (2), toluene (3), bromobenzene (4) and fluorobenzene (5)}. Compounds 2, 3, and 5 were crystallographically characterized, and in all cases the arene is π-coordinated. Solution NMR studies of 2-5 suggest that the binding preferences of the [(XA2)U(CH2SiMe3)]+ cation follow the order: toluene ≈ benzene > bromobenzene > fluorobenzene. Compounds 2-4 generated in C6H5R (R = H, Me or Br, respectively) showed no polymerization activity under 1 atm of ethylene. By contrast, 5 and 5-Th (the thorium analogue of 5) in fluorobenzene at 20 and 70 °C achieved ethylene polymerization activities between 16 800 and 139 200 g mol-1 h-1 atm-1, highlighting the extent to which common arene solvents such as toluene can suppress ethylene polymerization activity in sterically open f-element complexes. However, activation of [(XA2)An(CH2SiMe3)2] {M = U (1) or Th (1-Th)} with [Ph3C][B(C6F5)4] in n-alkane solvents did not afford an active polymerization catalyst due to catalyst decomposition, illustrating the critical role of PhX (X = H, Me, Br or F) coordination for alkyl cation stabilization. Gas phase DFT calculations, including fragment interaction calculations with energy decomposition and ETS-NOCV analysis, were carried out on the cationic portion of 2'-Th, 2', 3' and 5' (analogues of 2-Th, 2, 3 and 5 with hydrogen atoms in place of ligand backbone methyl and tert-butyl groups), providing insight into the nature of actinide-arene bonding, which decreases in strength in the order 2'-Th > 2' ≈ 3' > 5'.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




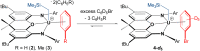



References
-
- Trifonov A. A. Lyubov D. M. Coord. Chem. Rev. 2017;340:10. doi: 10.1016/j.ccr.2016.09.013. - DOI
-
- Peng D. Yan X. Yu C. Zhang S. Li X. Polymer Chemistry. 2016;7:2601. doi: 10.1039/C6PY00040A. - DOI
-
- Altaf A. A. Badshah A. Khan N. Marwat S. Ali S. J. Coord. Chem. 2011;64:1815. doi: 10.1080/00958972.2011.568616. - DOI
-
- Burdett I. D. and Eisinger R. S., in Handbook of Industrial Polyethylene and Technology: Definitive Guide to Manufacturing, Properties, Processing, Applications and Markets, ed. M. A. Spalding and A. M. Chatterjee, Scrivener Publishing, Beverly, MA, 2018, ch. 3, p. 61
-
- Asua J. M., Polymer Reaction Engineering, Blackwell Publishing, Ames, Iowa, 2007
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

