Role of metalloproteases in the CD95 signaling pathways
- PMID: 36544756
- PMCID: PMC9760969
- DOI: 10.3389/fimmu.2022.1074099
Role of metalloproteases in the CD95 signaling pathways
Abstract
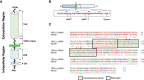
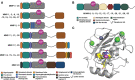
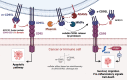
CD95L (also known as FasL or CD178) is a member of the tumor necrosis family (TNF) superfamily. Although this transmembrane ligand has been mainly considered as a potent apoptotic inducer in CD95 (Fas)-expressing cells, more recent studies pointed out its role in the implementation of non-apoptotic signals. Accordingly, this ligand has been associated with the aggravation of inflammation in different auto-immune disorders and in the metastatic occurrence in different cancers. Although it remains to decipher all key factors involved in the ambivalent role of this ligand, accumulating clues suggest that while the membrane bound CD95L triggers apoptosis, its soluble counterpart generated by metalloprotease-driven cleavage is responsible for its non-apoptotic functions. Nonetheless, the metalloproteases (MMPs and ADAMs) involved in the CD95L shedding, the cleavage sites and the different stoichiometries and functions of the soluble CD95L remain to be elucidated. To better understand how soluble CD95L triggers signaling pathways from apoptosis to inflammation or cell migration, we propose herein to summarize the different metalloproteases that have been described to be able to shed CD95L, their cleavage sites and the biological functions associated with the released ligands. Based on these new findings, the development of CD95/CD95L-targeting therapeutics is also discussed.
Keywords: ADAM; CD95L; MMP; cancer; cleavage; inflammation.
Copyright © 2022 Devel, Guedeney, Bregant, Chowdhury, Jean and Legembre.
Conflict of interest statement
PL and MJ are involved in patents protecting the use of CD95 or CD95L in chronic inflammatory disorders and cancers WO2014118317; WO2015189236; WO2015158810; WO2015104284; WO2017149012; WO2018130679. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

