Targeting 4-1BB and PD-L1 induces potent and durable antitumor immunity in B-cell lymphoma
- PMID: 36544785
- PMCID: PMC9762552
- DOI: 10.3389/fimmu.2022.1004475
Targeting 4-1BB and PD-L1 induces potent and durable antitumor immunity in B-cell lymphoma
Abstract
Introduction: Although PD-1/L1 mAb has demonstrated clinical benefits in certain cancer types, low response rate and resistance remain the main challenges for the application of these immune checkpoint inhibitors (ICIs). 4-1BB is a co-stimulator molecule expressed in T cells, which could enhance T cell proliferation and activation. Herein, the synergetic antitumor effect and underlying mechanism of 4-1BB agonist combined with PD-1/PD-L1 blockade were determined in B-cell lymphoma (BCL).
Methods: Subcutaneous transplantation BCL tumor models and metastasis models were established to evaluate the therapeutic effect of PD-L1 antibody and/or 4-1BB agonist in vivo. For the mechanistic study, RNA-seq was applied to analyze the tumor microenvironment and immune-related signal pathway after combination treatment. The level of IFN-γ, perforin, and granzyme B were determined by ELISA and Real-time PCR assays, while tumor-infiltrating T cells were measured by flow cytometry and immunohistochemical analysis. CD4/CD8 specific antibodies were employed to deplete the related T cells to investigate the role CD4+ and CD8+ T cells played in combination treatment.
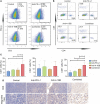
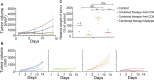
Results: Our results showed that combining anti-PD-L1 ICI and 4-1BB agonists elicited regression of BCL and significantly extended the survival of mice compared to either monotherapy. Co-targeting PD-L1 and 4-1BB preferentially promoted intratumoral cytotoxic lymphocyte infiltration and remodeled their function. RNA-sequence analysis uncovered a series of up-regulated genes related to the activation and proliferation of cytotoxic T lymphocytes, further characterized by increased cytokines including IFN-γ, granzyme B, and perforin. Furthermore, depleting CD8+ T cells not CD4+ T cells totally abrogated the antitumor efficacy, indicating the crucial function of the CD8+ T cell subset in the combination therapy.
Discussion: In summary, our findings demonstrated that 4-1BB agonistic antibody intensified the antitumor immunity of anti-PD-1/PD-L1 ICI via promoting CD8+ T cell infiltration and activation, providing a novel therapeutic strategy to BCL.
Keywords: 4-1BB agonist; B-cell lymphoma; CD8+ T cells; anti-PD-L1 ICI; combination therapy.
Copyright © 2022 Wang, Zhang, Xu, Nan, Fan, Zeng, Kwon and Ju.
Conflict of interest statement
BK is the founder and Chief Executive Officer of Eutilex Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. . Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov (2016) 6:827–37. doi: 10.1158/2159-8290.CD-15-1545 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

