Last resort beta-lactam antibiotics for treatment of New-Delhi Metallo-Beta-Lactamase producing Enterobacterales and other Difficult-to-Treat Resistance in Gram-negative bacteria: A real-life study
- PMID: 36544909
- PMCID: PMC9762507
- DOI: 10.3389/fcimb.2022.1048633
Last resort beta-lactam antibiotics for treatment of New-Delhi Metallo-Beta-Lactamase producing Enterobacterales and other Difficult-to-Treat Resistance in Gram-negative bacteria: A real-life study
Abstract
Introduction: Novel last resort beta-lactam antibiotics are now available for management of infections due to New-Delhi Metallo-Beta-Lactamase (NDM) producing Enterobacterales and non-fermenters with Difficult-to-Treat Resistance. However, data regarding the use of imipenem-cilastatin-relebactam (IMI-REL), cefiderocol (CFD) and ceftazidime-avibactam plus aztreonam (CAZ-AVI-ATM) are scarce in real-life settings. This study aimed to describe the use of last resort beta-lactam antibiotics, the microbiology and the outcome, in patients hospitalized in a tertiary hospital.
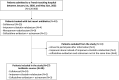
Methods: We conducted a monocentric observational cohort study from 2020/01/01, to 2022/08/31. We screened all patients admitted to Nimes University Hospital who have received ≥ 1 dose of last resort beta-lactam antibiotics during the study period, using the Pharmacy database. We included patients treated with IMI-REL, CFD and CAZ-AVI-ATM. The primary endpoint was the infection-free survival rate. We also calculated rates of microbiological and clinical cure, recurrent infection, death and adverse events.
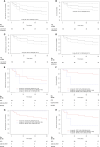
Results: Twenty-seven patients were included in the study and 30 treatment courses were analyzed: CFD (N=24; 80%), CAZ-AVI-ATM (N=3; 10%) and IMI-REL (N=3; 10%). Antibiotics were used in 21 males (70%) and 9 females (30%) with a median age at 65-year-old [50-73.5] and a median Charlson index at 1 [0-2]. Almost all the patients had ≥ 1 risk factor for carbapenem resistant bacteria, a half of them was hospitalized for severe COVID-19, and most of antibiotic courses (N=26; 87%) were associated with ICU admission. In the study population, the probability of infection-free survival at day-90 after last resort beta-lactam therapy initiation was 48.4% CI95% [33.2-70.5]. Clinical failure rate was at 30%, microbiological failure rate at 33% and mortality rate at 23%. Adverse events were documented in 5 antibiotic courses (17%). In details, P. aeruginosa were mainly treated with CFD and IMI-REL, S. maltophilia with CFD and CAZ-AVI-ATM, A. baumannii with CFD, and NDM producing-K. pneumoniae with CAZ-AVI-ATM and CFD. After a treatment course with CFD, CAZ-AVI-ATM and IMI-REL, the probability of infection-free survival was 48% CI95% [10.4-73.5], 33.3% CI95% [6.7-100], 66.7% CI95% [30-100], respectively.
Discussion/conclusion: Use of last resort beta-lactam antimicrobials in real-life settings was a safe and efficient therapeutic option for severe infections related to Gram-negative bacteria with Difficult-to-Treat Resistance.
Keywords: cefiderocol; ceftazidime-avibactam plus aztreonam; difficult to treat resistance; enterobacterales; imipenem-relebactam; metallo-beta-lactamase; new delhi metallo-beta-lactamase; pseudomonas aeruginosa.
Copyright © 2022 Larcher, Laffont-Lozes, Roger, Doncesco, Groul-Viaud, Martin, Loubet, Lavigne, Pantel and Sotto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Balandin B., Ballesteros D., Ruiz de Luna R., López-Vergara L., Pintado V., Sancho-González M., et al. (2021). Multicenter study of ceftolozane/tazobactam for treatment of pseudomonas aeruginosa infections in critically ill patients. Int. J. Antimicrob. Agents 57, 106270. doi: 10.1016/j.ijantimicag.2020.106270 - DOI - PubMed
-
- Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., et al. (2020). Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. doi: 10.1016/S1473-3099(20)30796-9 - DOI - PubMed
-
- Boulant T., Jousset A. B., Bonnin R. A., Barrail-Tran A., Borgel A., Oueslati S., et al. (2019). A 2.5-year within-patient evolution of pseudomonas aeruginosa isolates with In vivo acquisition of ceftolozane-tazobactam and ceftazidime-avibactam resistance upon treatment. Antimicrob. Agents Chemother. 63, e01637–e01619. doi: 10.1128/AAC.01637-19 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

