Clinically Significant Nonperfusion Areas on Widefield OCT Angiography in Diabetic Retinopathy
- PMID: 36545265
- PMCID: PMC9762190
- DOI: 10.1016/j.xops.2022.100241
Clinically Significant Nonperfusion Areas on Widefield OCT Angiography in Diabetic Retinopathy
Abstract
Purpose: To investigate the distribution of clinically significant nonperfusion areas (NPAs) on widefield OCT angiography (OCTA) images in patients with diabetes.
Design: Prospective, cross-sectional, observational study.
Participants: One hundred and forty-four eyes of 114 patients with diabetes.
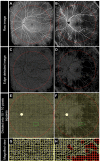
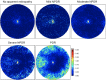
Methods: Nominal 20 × 23 mm OCTA images were obtained using a swept-source OCTA device (Xephilio OCT-S1), followed by the creation of en face images 20-mm (1614 pixels) in diameter centering on the fovea. The nonperfusion squares (NPSs) were defined as the 10 × 10 pixel squares without retinal vessels, and the ratio of eyes with the NPSs to all eyes in each square was referred to as the NPS ratio. The areas with probabilistic differences (APD) for proliferative diabetic retinopathy (PDR) and nonproliferative diabetic retinopathy (NPDR) (APD[PDR] and APD[NPDR]) were defined as sets of squares with higher NPS ratios in eyes with PDR and NPDR, respectively. The P ratio (NPSs within APD[PDR] but not APD[NPDR]/all NPSs) was also calculated.
Main outcome measures: The probabilistic distribution of the NPSs and the association with diabetic retinopathy (DR) severity.
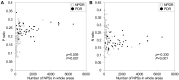
Results: The NPSs developed randomly in eyes with mild and moderate NPDR and were more prevalent in the extramacular areas and the temporal quadrant in eyes with severe NPDR and PDR. The APD(PDR) was distributed mainly in the extramacular areas, sparing the areas around the vascular arcades and radially peripapillary capillaries. The APD(PDR) contained retinal neovascularization more frequently than the non-APD(PDR) (P = 0.023). The P ratio was higher in eyes with PDR than in those with NPDR (P < 0.001). The multivariate analysis designated the P ratio (odds ratio, 8.293 × 107; 95% confidence interval, 6.529 × 102-1.053 × 1013; P = 0.002) and the total NPSs (odds ratio, 1.002; 95% confidence interval, 1.001-1.003; P < 0.001) as independent risk factors of PDR. Most eyes with NPDR and 4-2-1 rule findings of DR severity had higher P ratios but not necessarily greater NPS numbers.
Conclusions: The APD(PDR) is uniquely distributed on widefield OCTA images, and the NPA location patterns are associated with DR severity, independent of the entire area of NPAs.
Financial disclosures: Proprietary or commercial disclosure may be found after the references.
Keywords: APD, areas with probabilistic differences; DR, diabetic retinopathy; Diabetic retinopathy; FA, fluorescein angiography; IQR, interquartile range; IRMA, intraretinal microvascular abnormality; NPA, nonperfusion area; NPDR, nonproliferative diabetic retinopathy; NPS, nonperfusion square; NV, neovascularization; NVD, neovascularization of the disc; NVE, retinal neovascularization; Neovascularization; Nonperfusion areas; OCTA, OCT angiography; PDR, proliferative diabetic retinopathy; PRP, panretinal photocoagulation; RPC, radial peripapillary capillary; Semiautomatic quantification; VA, visual acuity; Widefield OCT angiography.
© 2022 Published by Elsevier Inc. on behalf of American Academy of Ophthalmology.
Figures



Similar articles
-
Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes With Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy.JAMA Ophthalmol. 2019 Jun 1;137(6):626-631. doi: 10.1001/jamaophthalmol.2019.0440. JAMA Ophthalmol. 2019. PMID: 30973596 Free PMC article. Clinical Trial.
-
Quantification of Nonperfusion Area in Montaged Widefield OCT Angiography Using Deep Learning in Diabetic Retinopathy.Ophthalmol Sci. 2021 May 12;1(2):100027. doi: 10.1016/j.xops.2021.100027. eCollection 2021 Jun. Ophthalmol Sci. 2021. PMID: 36249293 Free PMC article.
-
Interaction Between the Distribution of Diabetic Retinopathy Lesions and the Association of Optical Coherence Tomography Angiography Scans With Diabetic Retinopathy Severity.JAMA Ophthalmol. 2020 Dec 1;138(12):1291-1297. doi: 10.1001/jamaophthalmol.2020.4516. JAMA Ophthalmol. 2020. PMID: 33119083 Free PMC article.
-
Optical coherence tomography features of neovascularization in proliferative diabetic retinopathy: a systematic review.Int J Retina Vitreous. 2020 Jun 29;6:26. doi: 10.1186/s40942-020-00230-3. eCollection 2020. Int J Retina Vitreous. 2020. PMID: 32612851 Free PMC article. Review.
-
Update on Optical Coherence Tomography and Optical Coherence Tomography Angiography Imaging in Proliferative Diabetic Retinopathy.Diagnostics (Basel). 2021 Oct 11;11(10):1869. doi: 10.3390/diagnostics11101869. Diagnostics (Basel). 2021. PMID: 34679567 Free PMC article. Review.
Cited by
-
Severity Scale of Diabetic Macular Ischemia Based on the Distribution of Capillary Nonperfusion in OCT Angiography.Ophthalmol Sci. 2024 Sep 7;5(1):100603. doi: 10.1016/j.xops.2024.100603. eCollection 2025 Jan-Feb. Ophthalmol Sci. 2024. PMID: 39386056 Free PMC article.
-
Recent advances and applications of optical coherence tomography angiography in diabetic retinopathy.Front Endocrinol (Lausanne). 2025 Apr 16;16:1438739. doi: 10.3389/fendo.2025.1438739. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40309445 Free PMC article.
-
Inference of Capillary Nonperfusion Progression on Widefield OCT Angiography in Diabetic Retinopathy.Invest Ophthalmol Vis Sci. 2023 Oct 3;64(13):24. doi: 10.1167/iovs.64.13.24. Invest Ophthalmol Vis Sci. 2023. PMID: 37847225 Free PMC article.
References
-
- Antonetti D.A., Klein R., Gardner T.W. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. - PubMed
-
- Early Treatment Diabetic Retinopathy Study Research Group Fluorescein angiographic risk factors for progression of diabetic retinopathy. ETDRS report number 13. Ophthalmology. 1991;98(suppl 5):834–840. - PubMed
-
- Morino K., Murakami T., Dodo Y., et al. Characteristics of diabetic capillary nonperfusion in macular and extramacular white spots on optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60:1595–1603. - PubMed
-
- Kawai K., Uji A., Murakami T., et al. Image evaluation of artificial intelligence–supported optical coherence tomography angiography imaging using OCT-AI device in diabetic retinopathy. Retina. 2021;41:1730–1738. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

