The Twofold Role of Osteogenic Small Molecules in Parkinson's Disease Therapeutics: Crosstalk of Osteogenesis and Neurogenesis
- PMID: 36545269
- PMCID: PMC9763015
- DOI: 10.1155/2022/3813541
The Twofold Role of Osteogenic Small Molecules in Parkinson's Disease Therapeutics: Crosstalk of Osteogenesis and Neurogenesis
Abstract
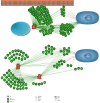
Deemed one of the most problematic neurodegenerative diseases in the elderly population, Parkinson's disease remains incurable to date. Ongoing diagnostic studies, however, have revealed that a large number of small molecule drugs that trigger the BMP2-Smad signaling pathway with an osteogenic nature may be effective in Parkinson's disease treatment. Although BMP2 and Smad1, 3, and 5 biomolecules promote neurite outgrowth and neuroprotection in dopaminergic cells as well, small molecules are quicker at crossing the BBB and reaching the damaged dopaminergic neurons located in the substantia nigra due to a molecular weight less than 500 Da. It is worth noting that osteogenic small molecules that inhibit Smurf1 phosphorylation do not offer therapeutic opportunities for Parkinson's disease; whereas, osteogenic small molecules that trigger Smad1, 3, and 5 phosphorylation may have strong therapeutic implications in Parkinson's disease by increasing the survival rate of dopaminergic cells and neuritogenesis. Notably, from a different perspective, it might be said that osteogenic small molecules can possibly put forth therapeutic options for Parkinson's disease by improving neuritogenesis and cell survival.
Copyright © 2022 Shima Tavakol.
Conflict of interest statement
The author indicates no potential conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

