Alkyltriphenylphosphonium turns naphthoquinoneimidazoles into potent membrane depolarizers against mycobacteria
- PMID: 36545436
- PMCID: PMC9749938
- DOI: 10.1039/d2md00251e
Alkyltriphenylphosphonium turns naphthoquinoneimidazoles into potent membrane depolarizers against mycobacteria
Abstract
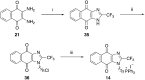
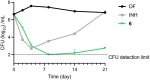
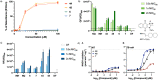
Due to its central role in energy generation and bacterial viability, mycobacterial bioenergetics is an attractive therapeutic target for anti-tuberculosis drug discovery. Building upon our work on antimycobacterial dioxonaphthoimidazoliums that were activated by a proximal positive charge and generated reactive oxygen species upon reduction by Type II NADH dehydrogenase, we herein studied the effect of a distal positive charge on the antimycobacterial activity of naphthoquinoneimidazoles by incorporating a trialkylphosphonium cation. The potency-enhancing properties of the linker length were affirmed by structure-activity relationship studies. The most active compound against M. tb H37Rv displayed good selectivity index (SI = 34) and strong bactericidal activity in the low micromolar range, which occurred through rapid bacterial membrane depolarization that resulted in depletion of intracellular ATP. Through this work, we demonstrated a switch of the scaffold's mode-of-action via relocation of positive charge while retaining its excellent antibacterial activity and selectivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



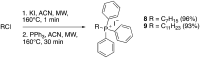




References
-
- Global Tuberculosis Report 2021, World Health Organization, Geneva, 2021. Licence: CC BY-NC-SA 3.0 IGO
-
- Gupta R. Espinal M. Int. J. Tuberc. Lung Dis. 2003;7:410–414. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

