Synthesis of chiral-at-metal rhodium complexes from achiral tripodal tetradentate ligands: resolution and application to enantioselective Diels-Alder and 1,3-dipolar cycloadditions
- PMID: 36545596
- PMCID: PMC9717581
- DOI: 10.1039/d2ra06982b
Synthesis of chiral-at-metal rhodium complexes from achiral tripodal tetradentate ligands: resolution and application to enantioselective Diels-Alder and 1,3-dipolar cycloadditions
Abstract
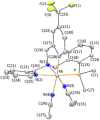
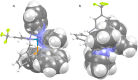
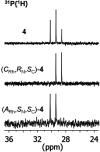
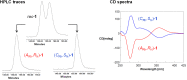
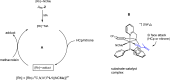
An improved synthesis of the racemic rhodium compound [RhCl2(κ4 C,N,N',P-L1)] (1) containing an achiral tripodal tetradentate ligand is reported. Their derived solvate complexes [Rh(κ4 C,N,N',P-L1)(Solv)2][SbF6]2 (Solv = NCMe, 2; H2O, 3) are resolved into their two enantiomers. Complexes 2 and 3 catalyze the Diels-Alder (DA) reaction between methacrolein and cyclopentadiene and the 1,3-dipolar cycloaddition reaction between methacrolein and the nitrone N-benzylidenphenylamine-N-oxide. When enantiopure (A Rh,R N)-2 was employed as the catalyst, enantiomeric ratios >99/1, in the R at C2 adduct, and up to 94/6, in the 3,5-endo isomer, were achieved in the DA reaction and in the 1,3-dipolar cycloaddition reaction, respectively. A plausible catalytic cycle that accounts for the origin of the observed enantioselectivity is proposed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

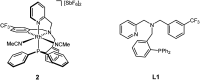

References
-
- Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer, New York, 1999, vol. I–III
- Catalytic Asymmetric Synthesis, ed. I. Ojima, VCH, Weinheim, 2000
- Noyori R., Asymmetric Catalysis in Organic Synthesis, John Willey and Sons, New York, 1994
- Walsh P. J. and Kozlowski M. C., Fundamentals of Asymmetric Catalysis, University Science Books, Sausalito, 2009
-
- Brunner H. Acc. Chem. Res. 1979;12:250–257. doi: 10.1021/ar50139a005. - DOI
-
- Werner A. Ber. Dtsch. Chem. Ges. 1911;44:1887–1898. doi: 10.1002/cber.19110440297. - DOI
-
- Gong L. Wenzel M. Meggers E. Acc. Chem. Res. 2013;46:2635–2644. doi: 10.1021/ar400083u. - DOI - PubMed
- Meggers E. Eur. J. Inorg. Chem. 2011:2911–2926. doi: 10.1002/ejic.201100327. - DOI
- Meggers E. Chem.–Eur. J. 2010;16:752–758. doi: 10.1002/chem.200902652. - DOI - PubMed
- Fontecabe M. Hamelin O. Ménage S. Top. Organomet. Chem. 2005;15:271–288. doi: 10.1007/b136353. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

