Antibody response durability following three-dose coronavirus disease 2019 vaccination in people with HIV receiving suppressive antiretroviral therapy
- PMID: 36545783
- PMCID: PMC9994797
- DOI: 10.1097/QAD.0000000000003469
Antibody response durability following three-dose coronavirus disease 2019 vaccination in people with HIV receiving suppressive antiretroviral therapy
Abstract
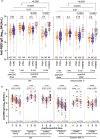
Background: Limited data exist regarding longer term antibody responses following three-dose coronavirus disease 2019 (COVID-19) vaccination, and the impact of a first SARS-CoV-2 infection during this time, in people with HIV (PWH) receiving suppressive antiretroviral therapy (ART). We quantified wild-type-specific, Omicron BA.1-specific and Omicron BA.5-specific responses up to 6 months post-third dose in 64 PWH and 117 controls who remained COVID-19-naive or experienced their first SARS-CoV-2 infection during this time.
Design: Longitudinal observational cohort.
Methods: We quantified wild-type-specific and Omicron-specific anti-Spike receptor-binding domain IgG concentrations, ACE2 displacement activities and live virus neutralization at 1, 3 and 6 months post-third vaccine dose.
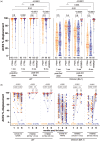
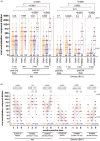
Results: Third doses boosted all antibody measures above two-dose levels, but BA.1-specific responses remained significantly lower than wild-type-specific ones, with BA.5-specific responses lower still. Serum IgG concentrations declined at similar rates in COVID-19-naive PWH and controls post-third dose (median wild-type-specific and BA.1-specific half-lives were between 66 and 74 days for both groups). Antibody function also declined significantly yet comparably between groups: 6 months post-third dose, BA.1-specific neutralization was undetectable in more than 80% of COVID-19 naive PWH and more than 90% of controls. Breakthrough SARS-CoV-2 infection boosted antibody concentrations and function significantly above vaccine-induced levels in both PWH and controls, though BA.5-specific neutralization remained significantly poorer than BA.1 even post-breakthrough.
Conclusion: Following three-dose COVID-19 vaccination, antibody response durability in PWH receiving ART is comparable with controls. PWH also mounted strong responses to breakthrough infection. Due to temporal response declines, however, COVID-19-naive individuals, regardless of HIV status, would benefit from a fourth dose within 6 months of their third.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures

Comment in
-
Effectiveness of COVID-19 primary and booster vaccination in HIV-infected individuals.AIDS. 2023 Apr 1;37(5):837-839. doi: 10.1097/QAD.0000000000003492. AIDS. 2023. PMID: 36919787 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

