Reduced platelet glycoprotein Ibα shedding accelerates thrombopoiesis and COX-1 recovery: implications for aspirin dosing regimen
- PMID: 36546455
- PMCID: PMC10071111
- DOI: 10.3324/haematol.2022.281006
Reduced platelet glycoprotein Ibα shedding accelerates thrombopoiesis and COX-1 recovery: implications for aspirin dosing regimen
Abstract
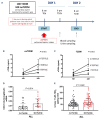
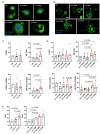
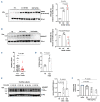
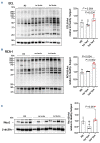
Cardiovascular (CV) disease prevention with low-dose aspirin can be less effective in patients with a faster recovery of platelet (PLT) cyclooxygenase (COX)-1 activity during the 24-hour dosing interval. We previously showed that incomplete suppression of TXA2 over 24 hours can be rescued by a twice daily aspirin regimen. Here we show that reduced PLT glycoprotein (GP)Ibα shedding characterizes patients with accelerated COX-1 recovery and may contribute to higher thrombopoietin (TPO) production and higher rates of newly formed PLT, escaping aspirin inhibition over 24 hours. Two hundred aspirin-treated patients with high CV risk (100 with type 2 diabetes mellitus) were stratified according to the kinetics of PLT COX-1 activity recovery during the 10- to 24-hour dosing interval. Whole proteome analysis showed that PLT from patients with accelerated COX-1 recovery were enriched in proteins involved in cell survival, inhibition of apoptosis and cellular protrusion formation. In agreement, we documented increased plasma TPO, megakaryocyte maturation and proplatelet formation, and conversely increased PLT galactose and reduced caspase 3, phosphatidylserine exposure and ADAM17 activation, translating into diminished GPIbα cleavage and glycocalicin (GC) release. Treatment of HepG2 cells with recombinant GC led to a dose-dependent reduction of TPO mRNA in the liver, suggesting that reduced GPIbα ectodomain shedding may unleash thrombopoiesis. A cluster of clinical markers, including younger age, non-alcoholic fatty liver disease, visceral obesity and higher TPO/GC ratio, predicted with significant accuracy the likelihood of faster COX-1 recovery and suboptimal aspirin response. Circulating TPO/GC ratio, reflecting a dysregulation of PLT lifespan and production, may provide a simple tool to identify patients amenable to more frequent aspirin daily dosing.
Figures



References
-
- Davì G, Patrono C. Mechanisms of disease: platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482-2494. - PubMed
-
- Davì G, Catalano I, Averna M, et al. . Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med. 1990;322(25):1769-1774. - PubMed
-
- Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015;120:28-39. - PubMed
-
- Patrono C, Rodríguez LAG, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373-2383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

