Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C
- PMID: 36546659
- PMCID: PMC9908297
- DOI: 10.1056/NEJMoa2212419
Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C
Abstract
Background: Adagrasib, an oral small-molecule inhibitor of mutant KRAS G12C protein, has shown clinical activity in pretreated patients with several tumor types, including colorectal cancer. Preclinical studies suggest that combining a KRAS G12C inhibitor with an epidermal growth factor receptor antibody could be an effective clinical strategy.
Methods: In this phase 1-2, open-label, nonrandomized clinical trial, we assigned heavily pretreated patients with metastatic colorectal cancer with mutant KRAS G12C to receive adagrasib monotherapy (600 mg orally twice daily) or adagrasib (at the same dose) in combination with intravenous cetuximab once a week (with an initial loading dose of 400 mg per square meter of body-surface area, followed by a dose of 250 mg per square meter) or every 2 weeks (with a dose of 500 mg per square meter). The primary end points were objective response (complete or partial response) and safety.
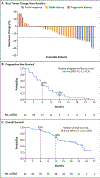
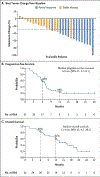
Results: As of June 16, 2022, a total of 44 patients had received adagrasib, and 32 had received combination therapy with adagrasib and cetuximab, with a median follow-up of 20.1 months and 17.5 months, respectively. In the monotherapy group (43 evaluable patients), a response was reported in 19% of the patients (95% confidence interval [CI], 8 to 33). The median response duration was 4.3 months (95% CI, 2.3 to 8.3), and the median progression-free survival was 5.6 months (95% CI, 4.1 to 8.3). In the combination-therapy group (28 evaluable patients), the response was 46% (95% CI, 28 to 66). The median response duration was 7.6 months (95% CI, 5.7 to not estimable), and the median progression-free survival was 6.9 months (95% CI, 5.4 to 8.1). The percentage of grade 3 or 4 treatment-related adverse events was 34% in the monotherapy group and 16% in the combination-therapy group. No grade 5 adverse events were observed.
Conclusions: Adagrasib had antitumor activity in heavily pretreated patients with metastatic colorectal cancer with mutant KRAS G12C, both as oral monotherapy and in combination with cetuximab. The median response duration was more than 6 months in the combination-therapy group. Reversible adverse events were common in the two groups. (Funded by Mirati Therapeutics; KRYSTAL-1 ClinicalTrials.gov number, NCT03785249.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Precision oncology for KRASG12C-mutant colorectal cancer.Nat Rev Clin Oncol. 2023 Jun;20(6):355-356. doi: 10.1038/s41571-023-00748-z. Nat Rev Clin Oncol. 2023. PMID: 36914743 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
