Babchi Oil-Based Nanoemulsion Hydrogel for the Management of Psoriasis: A Novel Energy Economic Approach Employing Biosurfactants
- PMID: 36547285
- PMCID: PMC9777791
- DOI: 10.3390/gels8120761
Babchi Oil-Based Nanoemulsion Hydrogel for the Management of Psoriasis: A Novel Energy Economic Approach Employing Biosurfactants
Abstract
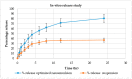
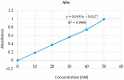
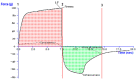
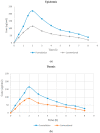
The current research aimed to assess the Babchi oil nanoemulsion-based hydrogel prepared using biosurfactants through a low-energy emulsification process for the topical management of psoriasis. The emulsification capacity and solubilities of many nanoemulsion constituents such as surfactants, co-surfactants, and oil were considered to determine the range of concentration of the constituents. Pseudoternary phase diagrams were created using the method of titration. Nanoemulgel structure, morphology, micromeritics, conductivity, and viscosity were all optimized. The assessment of the Babchi oil nanoemulgel included particle size, polydispersity index (PDI), drug content, pH, spreadability, rheological management, ex vivo drug study, 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging ability, in vitro drug release, release kinetics, and dermatokinetics. The selected ratios of the surfactant mixture (Smix) taken were 3:1. The entrapment efficiency estimated was 91.298%. The zeta potential of Babchi oil was observed to be -24.93 mV at 25 °C with water as a dispersant, viscosity as 0.887 cP, and material absorption as 0.01 nm. The size distribution of the particle was 108 nm by the intensity and the conductivity observed was 0.03359 mS/cm. The cumulative amount of Babchi oil penetrated and fluxed by nanoemulgel was considered larger (p ≤ 0.05) than the conventional formulations. Skin retention was observed to be good with decreased lag time. The formulation followed the Higuchi Korsmeyer for Fickian Peppas model for in vitro drug release studies. The oil was most effective on the epidermal layer of the skin for treatment. It was established that the Babchi oil nanoemulgel formulation had superior permeability capabilities for topical and transdermal administration and is a viable alternative to traditional formulations.
Keywords: Babchi oil; low energy emulsification; nanoemulgel; nanoemulsion-based hydrogel; psoriasis.
Conflict of interest statement
There is no conflict of interest.
Figures







References
-
- Salim N., Ahmad N., Musa S.H., Hashim R., Tadros T.F., Basri M. Nanoemulsion as a Topical Delivery System of Antipsoriatic Drugs. RSC Adv. 2016;6:6234–6250. doi: 10.1039/C5RA14946K. - DOI
-
- Sarkar S. A Treatise on Topical Corticosteroid in Dermatology. Indian J. Dermatol. 2018;63:530. doi: 10.4103/IJD.IJD_297_18. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

