Complexes of Cu-Polysaccharide of a Marine Red Microalga Produce Spikes with Antimicrobial Activity
- PMID: 36547934
- PMCID: PMC9783634
- DOI: 10.3390/md20120787
Complexes of Cu-Polysaccharide of a Marine Red Microalga Produce Spikes with Antimicrobial Activity
Abstract
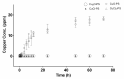
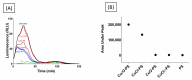
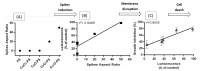
Metal-polysaccharides have recently raised significant interest due to their multifunctional bioactivities. The antimicrobial activity of a complex of Cu2O with the sulfated polysaccharide (PS) of the marine red microalga Porphyridium sp. was previously attributed to spikes formed on the complex surface (roughness). This hypothesis was further examined here using other Cu-PS complexes (i.e., monovalent-Cu2O, CuCl and divalent-CuO, CuCl2). The nanostructure parameters of the monovalent complexes, namely, longer spikes (1000 nm) and greater density (2000-5000 spikes/µm2) were found to be related to the superior inhibition of microbial growth and viability and biofilm formation. When Escherichia coli TV1061, used as a bioluminescent test organism, was exposed to the monovalent Cu-PS complexes, enhanced bioluminescence accumulation was observed, probably due to membrane perforation by the spikes on the surface of the complexes and consequent cytoplasmic leakage. In addition, differences were found in the surface chemistry of the monovalent and divalent Cu-PS complexes, with the monovalent Cu-PS complexes exhibiting greater stability (ζ-potential, FTIR spectra, and leaching out), which could be related to spike formation. This study thus supports our hypothesis that the spikes protruding from the monovalent Cu-PS surfaces, as characterized by their aspect ratio, are responsible for the antimicrobial and antibiofilm activities of the complexes.
Keywords: antimicrobial activities; biomaterials; metal complexes; red microalgae; spike formation; sulfated polysaccharides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Lapidot M., Shrestha R.P., Weinstein Y., Arad S. Red Microalgae: From Basic Know-How to Biotechnology. Springer; Berlin/Heidelberg, Germany: 2010. pp. 205–225.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

