Antidrug Antibodies to Tumor Necrosis Factor α Inhibitors in Patients With Noninfectious Uveitis
- PMID: 36547953
- PMCID: PMC9936342
- DOI: 10.1001/jamaophthalmol.2022.5584
Antidrug Antibodies to Tumor Necrosis Factor α Inhibitors in Patients With Noninfectious Uveitis
Abstract
Importance: Tumor necrosis factor inhibitors (TNFis) can induce antidrug antibody (ADA) formation and loss of therapeutic response. However, the utility of ADA testing and the association between ADAs and treatment response in patients with noninfectious uveitis (NIU) is not well understood.
Objective: To assess the frequency of ADAs and their association with drug levels and clinical response in patients with NIU treated with adalimumab or infliximab.
Design, setting, and participants: This retrospective cross-sectional study included patients diagnosed with NIU who received adalimumab or infliximab and underwent testing for serum drug level and ADAs at the National Eye Institute from September 2017 to July 2021.
Exposures: Serum drug level testing with reflex testing for ADA levels was performed.
Main outcomes and measures: The main outcome was the association between drug levels and ADAs, clinical response, and concurrent antimetabolite use in patients treated with TNFis for NIU.
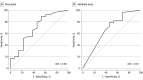
Results: Of 54 patients included in the study, 42 received adalimumab (mean [SD] age, 43.6 [19.6] years; 25 [59.5%] female) and 12 received infliximab (mean [SD] age, 42.7 [20.4] years; 7 [58.3%] male). In the adalimumab group, mean (SD) drug level was 9.72 (6.82) μg/mL, mean (SD) ADA level was 84.2 (172.9) arbitrary units/mL, and ADA frequency was 35.7% (15 of 42 patients). Mean drug level was lower in those with ADAs compared with those without ADAs (mean [SD], 2.8 [2.6] μg/mL vs 13.6 [5.2] μg/mL; difference: 10.8 μg/mL; 95% CI, 8.3-13.2 μg/mL; P < .001). There was a higher mean drug level with concurrent antimetabolite use compared with monotherapy (mean [SD], 11.0 [7.3] μg/mL vs 6.8 [4.5] μg/mL; difference: -4.2 μg/mL; 95% CI, -8.7 to 0.2 μg/mL; P = .06). Multivariable modeling showed that a 1-arbitrary unit increase in ADAs was associated with a -0.02 μg/mL (95% CI, -0.01 to -0.34 μg/mL) difference in mean drug level (P < .001). Favorable clinical response was associated with a threshold drug level above 2.7 μg/mL or an antibody level below 15.2 μg/mL. The mean (SD) drug level in the infliximab group was 27.02 (18.15) μg/mL, and no ADAs were detected.
Conclusions and relevance: In this study, 35.7% of adalimumab-treated patients with NIU had ADAs. The presence of ADAs was associated with lower drug levels, and higher ADA levels were associated with increased risk of TNFi treatment failure. Although limited by the retrospective design, our results suggest that therapeutic drug monitoring may be considered among patients experiencing therapy failure to help exclude ADAs as a potential cause of treatment failure.
Conflict of interest statement
Figures
Comment in
-
Therapeutic Drug Monitoring in Noninfectious Uveitis.JAMA Ophthalmol. 2023 Feb 1;141(2):156-158. doi: 10.1001/jamaophthalmol.2022.5685. JAMA Ophthalmol. 2023. PMID: 36547991 No abstract available.
References
-
- Nguyen QD, Merrill PT, Jaffe GJ, et al. . Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet. 2016;388(10050):1183-1192. doi:10.1016/S0140-6736(16)31339-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials