Single-Standard Quantification Strategy for Lignin Dimers by Supercritical Fluid Chromatography with Charged Aerosol Detection
- PMID: 36548212
- PMCID: PMC9850414
- DOI: 10.1021/acs.analchem.2c04383
Single-Standard Quantification Strategy for Lignin Dimers by Supercritical Fluid Chromatography with Charged Aerosol Detection
Abstract
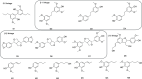
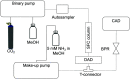
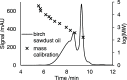
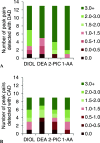
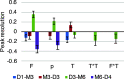
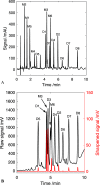
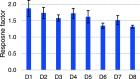
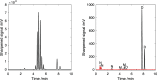
The increased interest in utilizing lignin as a feedstock to produce various aromatic compounds requires advanced chemical analysis methods to provide qualitative and quantitative characterization of lignin samples along different technology streamlines. However, due to the lack of commercially available chemical standards, routine quantification of industrially relevant lignin oligomers in complex lignin samples remains a challenge. This study presents a novel method for universal quantification of lignin dimers based on supercritical fluid chromatography with charged aerosol detection (CAD). A series of lignin-derived dimeric compounds that have been reported from reductive catalytic fractionation (RCF) were synthesized and used as standards. The applicability of using linear regression instead of quadratic calibration curves was evaluated over a concentration range of 15-125 mg/L, demonstrating that the former calibration method is as appropriate as the latter. The response factors of lignin dimeric compounds were compared to assess the uniformity of the CAD signal, revealing that the CAD response for the tested lignin dimers did not differ substantially. It was also found that the response factors were not dependent on the number of methoxy groups or linkage motifs, ultimately enabling the use of only one calibrant for these compounds. The importance of chromatographic peak resolution in CAD was stressed, and the use of a digital peak sharpening technique was adopted and applied to address this challenge. The developed method was verified and used for the quantification of lignin dimers in an oil obtained by a RCF of birch sawdust.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Abejón R.; Pérez-Acebo H.; Clavijo L. Alternatives for Chemical and Biochemical Lignin Valorization: Hot Topics from a Bibliometric Analysis of the Research Published during the 2000-2016 Period. Processes 2018, 6, 98.10.3390/pr6080098. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

