Modifying the ICP pulse wave: effects on parenchymal blood flow pulsatility
- PMID: 36548513
- PMCID: PMC9886344
- DOI: 10.1152/japplphysiol.00401.2022
Modifying the ICP pulse wave: effects on parenchymal blood flow pulsatility
Abstract
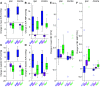
Pulsation of the cerebral blood flow (CBF) produces intercranial pressure (ICP) waves. The aim of this study is to determine whether externally modifying ICP pulsatility alters parenchymal blood flow pulsatility. A cardiac-gated inflatable device was inserted in the lateral epidural space of 12 anesthetized canines (canis familiaris) and used to cause reduction, inversion, and augmentation of the ICP pulse. CBF in each hemisphere was measured using laser Doppler velocimetry. A significant increase in both mean CBF and its amplitude was observed for reduction as well as inversion of the ICP pulse, with larger changes observed for the inversion protocol. Significant increases in the mean CBF were also observed ipsilaterally for the augmentation protocol together with indications of reduced CBF amplitude contralaterally. External alteration of the ICP pulse thus caused significant changes in parenchymal blood flow pulsatility. The inverse relationship between the ICP and CBF amplitude suggests that the changes did not occur via modification of the intracranial Windkessel mechanism. Thus, the effects likely occurred in the low-pressure vessels, i.e., capillaries and/or venules, rather than the high-pressure arteries. Future MRI studies are however required to map and quantify the effects on global cerebral blood flow.NEW & NOTEWORTHY This study demonstrated that external modification of ICP pulsatility, using a cardiac-gated inflatable device implanted epidurally in canines, alters brain tissue blood flow pulsatility. Specifically, decreasing systolic ICP increased blood flow pulsatility in brain tissue. The results suggest that the altered CBF pulsatility is unlikely to depend on modification of the Windkessel effect on the feeding arterial system but was rather an effect directly on tissue and the lower pressure distal vessels.
Keywords: cardiac-gated inflatable device; cerebral blood flow; experimental model; intracranial pressure; intracranial pulsatility.
Conflict of interest statement
M.G.L. and S.M.D. are listed as inventors on two patents regarding the device presented in this paper, but do not have any conflict of interest otherwise. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures






References
-
- Mitchell GF, Van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility-Reykjavik Study. Brain 134: 3398–3407, 2011. doi:10.1093/brain/awr253. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

