A Novel Protocol for the Synthesis of 1,2,4-Oxadiazoles Active against Trypanosomatids and Drug-Resistant Leukemia Cell Lines
- PMID: 36548658
- PMCID: PMC9787607
- DOI: 10.3390/tropicalmed7120403
A Novel Protocol for the Synthesis of 1,2,4-Oxadiazoles Active against Trypanosomatids and Drug-Resistant Leukemia Cell Lines
Abstract
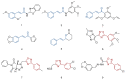
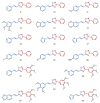
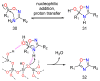
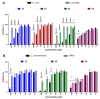
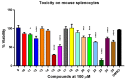
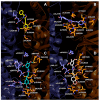
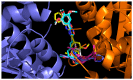
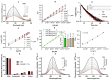
Cancer and parasitic diseases, such as leishmaniasis and Chagas disease, share similarities that allow the co-development of new antiproliferative agents as a strategy to quickly track the discovery of new drugs. This strategy is especially interesting regarding tropical neglected diseases, for which chemotherapeutic alternatives are extremely outdated. We designed a series of (E)-3-aryl-5-(2-aryl-vinyl)-1,2,4-oxadiazoles based on the reported antiparasitic and anticancer activities of structurally related compounds. The synthesis of such compounds led to the development of a new, fast, and efficient strategy for the construction of a 1,2,4-oxadiazole ring on a silica-supported system under microwave irradiation. One hit compound (23) was identified during the in vitro evaluation against drug-sensitive and drug-resistant chronic myeloid leukemia cell lines (EC50 values ranging from 5.5 to 13.2 µM), Trypanosoma cruzi amastigotes (EC50 = 2.9 µM) and Leishmania amazonensis promastigotes (EC50 = 12.2 µM) and amastigotes (EC50 = 13.5 µM). In silico studies indicate a correlation between the in vitro activity and the interaction with tubulin at the colchicine binding site. Furthermore, ADMET in silico predictions indicate that the compounds possess a high druggability potential due to their physicochemical, pharmacokinetic, and toxicity profiles, and for hit 23, it was identified by multiple spectroscopic approaches that this compound binds with human serum albumin (HSA) via a spontaneous ground-state association with a moderate affinity driven by entropically and enthalpically energies into subdomain IIA (site I) without significantly perturbing the secondary content of the protein.
Keywords: Leishmania amazonensis; Trypanosoma cruzi; anticancer; chagas disease; chronic myeloid leukemia; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization Research Priorities for Chagas Disease, Human African Trypanosomiasis and Leishmaniasis. [(accessed on 29 September 2022)]. Available online: https://apps.who.int/iris/handle/10665/77472. - PubMed
-
- World Health Organization Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Diseases (No. WHO/HTM/NTD/2013.1) [(accessed on 29 September 2022)]. Available online: https://apps.who.int/iris/handle/10665/77950.
-
- Espinosa A.V., Costa D.D.S., Tunes L.G., Monte-Neto R.L.D., Grazul R.M., de Almeida M.V., Silva H. Anticancer and antileishmanial in vitro activity of gold (I) complexes with 1, 3, 4-oxadiazole-2 (3H)-thione ligands derived from δ-D-gluconolactone. Chem. Biol. Drug Des. 2021;97:41–50. doi: 10.1111/cbdd.13757. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

