Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial
- PMID: 36548927
- PMCID: PMC10115554
- DOI: 10.1200/JCO.22.01725
Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial
Abstract
Purpose: Epcoritamab is a subcutaneously administered CD3xCD20 T-cell-engaging, bispecific antibody that activates T cells, directing them to kill malignant CD20+ B cells. Single-agent epcoritamab previously demonstrated potent antitumor activity in dose escalation across B-cell non-Hodgkin lymphoma subtypes.
Patients and methods: In the dose-expansion cohort of a phase I/II study (ClinicalTrials.gov identifier: NCT03625037), adults with relapsed or refractory CD20+ large B-cell lymphoma and at least two prior therapy lines (including anti-CD20 therapies) received subcutaneous epcoritamab in 28-day cycles (once weekly step-up doses in weeks 1-3 of cycle 1, then full doses once weekly through cycle 3, once every 2 weeks in cycles 4-9, and once every 4 weeks in cycle 10 and thereafter) until disease progression or unacceptable toxicity. The primary end point was overall response rate by the independent review committee.
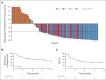
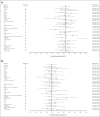
Results: As of January 31, 2022, 157 patients were treated (median age, 64 years [range, 20-83]; median of three [range, 2-11] prior therapy lines; primary refractory disease: 61.1%; prior chimeric antigen receptor (CAR) T-cell exposure: 38.9%). At a median follow-up of 10.7 months, the overall response rate was 63.1% (95% CI, 55.0 to 70.6) and the complete response rate was 38.9% (95% CI, 31.2 to 46.9). The median duration of response was 12.0 months (among complete responders: not reached). Overall and complete response rates were similar across key prespecified subgroups. The most common treatment-emergent adverse events were cytokine release syndrome (49.7%; grade 1 or 2: 47.1%; grade 3: 2.5%), pyrexia (23.6%), and fatigue (22.9%). Immune effector cell-associated neurotoxicity syndrome occurred in 6.4% of patients with one fatal event.
Conclusion: Subcutaneous epcoritamab resulted in deep and durable responses and manageable safety in highly refractory patients with large B-cell lymphoma, including those with prior CAR T-cell exposure.
Conflict of interest statement
Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell–Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Catherine Thieblemont
Tycel Phillips
Herve Ghesquieres
Chan Y. Cheah
Michael Roost Clausen
David Cunningham
Tatyana Feldman
Robin Gasiorowski
Wojciech Jurczak
Tae Min Kim
David John Lewis
Marjolein van der Poel
Mariana Cota Stirner
Nurgul Kilavuz
Christopher Chiu
Menghui Chen
Mariana Sacchi
Brian Elliott
Tahamtan Ahmadi
Martin Hutchings
Pieternella J. Lugtenburg
No other potential conflicts of interest were reported.
Figures
Comment in
-
Epcoritamab is active in large B cell lymphomas.Nat Rev Clin Oncol. 2023 Mar;20(3):138. doi: 10.1038/s41571-023-00730-9. Nat Rev Clin Oncol. 2023. PMID: 36646813 No abstract available.
References
-
- Wienand K, Chapuy B: Molecular classification of aggressive lymphomas-past, present, future. Hematol Oncol 39:24-30, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

