Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease
- PMID: 36549300
- PMCID: PMC9839529
- DOI: 10.1016/j.chom.2022.11.015
Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease
Abstract
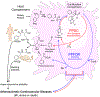
Recent studies show gut microbiota-dependent metabolism of dietary phenylalanine into phenylacetic acid (PAA) is critical in phenylacetylglutamine (PAGln) production, a metabolite linked to atherosclerotic cardiovascular disease (ASCVD). Accordingly, microbial enzymes involved in this transformation are of interest. Using genetic manipulation in selected microbes and monocolonization experiments in gnotobiotic mice, we identify two distinct gut microbial pathways for PAA formation; one is catalyzed by phenylpyruvate:ferredoxin oxidoreductase (PPFOR) and the other by phenylpyruvate decarboxylase (PPDC). PPFOR and PPDC play key roles in gut bacterial PAA production via oxidative and non-oxidative phenylpyruvate decarboxylation, respectively. Metagenomic analyses revealed a significantly higher abundance of both pathways in gut microbiomes of ASCVD patients compared with controls. The present studies show a role for these two divergent microbial catalytic strategies in the meta-organismal production of PAGln. Given the numerous links between PAGln and ASCVD, these findings will assist future efforts to therapeutically target PAGln formation in vivo.
Keywords: Atherosclerotic cardiovascular disease; gut microbes; phenylacetic acid; phenylacetylglutamine; phenylacetylglycine; phenylalanine; phenylpyruvate decarboxylase; phenylpyruvate:ferredoxin oxidoreductase.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.L.H., Z.W., and J.T.A. report being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and/or therapeutics and being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland HeartLab, a wholly owned subsidiary of Quest Diagnostics, P&G, and Zehna Therapeutics. S.L.H. and J.T.A. report being paid consultants for Zehna Therapeutics. S.L.H. also reports having received research funds from P&G, Roche Diagnostics, and Zehna Therapeutics. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






Comment in
-
Meta-organismal metabolism in cardiovascular disease.Cell Host Microbe. 2023 Jan 11;31(1):1-2. doi: 10.1016/j.chom.2022.12.007. Cell Host Microbe. 2023. PMID: 36634617
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

