Immunogenicity and safety of coadministration of COVID-19 and influenza vaccination
- PMID: 36549716
- PMCID: PMC9773493
- DOI: 10.1183/13993003.01390-2022
Immunogenicity and safety of coadministration of COVID-19 and influenza vaccination
Abstract
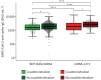
Coadministration of seasonal quadrivalent influenza and COVID-19 booster vaccination is safe and does not increase vaccine-related side-effects, but may limit anti-SARS-CoV-2 antibody formation https://bit.ly/3uKFUie
Conflict of interest statement
Conflict of interest: M. Prelog received honoraria for presentations at scientific meetings from Abbvie, Chugai-Roche, GSK, Janssen, Novartis, MSD, Pfizer, Sanofi and SOBI, received honoraria for advisory boards from Abbvie, GSK, Janssen, Novartis, Pfizer and BioNTech, received travel scholarships from Chugai-Roche, GSK, Novartis and Pfizer, received financial support for conduction of investigator-initiated research from Baxter, Chugai-Roche, GSK, Novartis, MSD, Pfizer and SOBI; M. Prelog declares to have no conflict of interest regarding the manuscript. M. Krone receives honoraria from GSK and Pfizer, outside the submitted work. All other authors declare no potential conflicts of interest.
Figures
References
-
- Izikson R, Bolduc D, Bourron JS, et al. . Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir Med 2022; 10: 392–402. doi:10.1016/S2213-2600(21)00557-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous