TIGIT blockade repolarizes AML-associated TIGIT+ M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis
- PMID: 36549780
- PMCID: PMC9791419
- DOI: 10.1136/jitc-2022-004794
TIGIT blockade repolarizes AML-associated TIGIT+ M2 macrophages to an M1 phenotype and increases CD47-mediated phagocytosis
Abstract
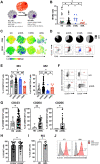
Background: Leukemia-associated macrophages (LAMs) represent an important cell population within the tumor microenvironment, but little is known about the phenotype, function, and plasticity of these cells. The present study provides an extensive characterization of macrophages in patients with acute myeloid leukemia (AML).
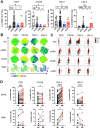
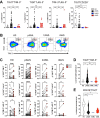
Methods: The phenotype and expression of coregulatory markers were assessed on bone marrow (BM)-derived LAM populations, using multiparametric flow cytometry. BM and blood aspirates were obtained from patients with newly diagnosed acute myeloid leukemia (pAML, n=59), patients in long-term remission (lrAML, n=8), patients with relapsed acute myeloid leukemia (rAML, n=7) and monocyte-derived macrophages of the blood from healthy donors (HD, n=17). LAM subpopulations were correlated with clinical parameters. Using a blocking anti-T-cell immunoreceptor with Ig and ITIM domains (TIGIT) antibody or mouse IgG2α isotype control, we investigated polarization, secretion of cytokines, and phagocytosis on LAMs and healthy monocyte-derived macrophages in vitro.
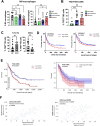
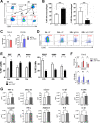
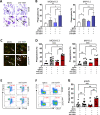
Results: In pAML and rAML, M1 LAMs were reduced and the predominant macrophage population consisted of immunosuppressive M2 LAMs defined by expression of CD163, CD204, CD206, and CD86. M2 LAMs in active AML highly expressed inhibitory receptors such as TIGIT, T-cell immunoglobulin and mucin-domain containing-3 protein (TIM-3), and lymphocyte-activation gene 3 (LAG-3). High expression of CD163 was associated with a poor overall survival (OS). In addition, increased frequencies of TIGIT+ M2 LAMs were associated with an intermediate or adverse risk according to the European Leukemia Network criteria and the FLT3 ITD mutation. In vitro blockade of TIGIT shifted the polarization of primary LAMs or peripheral blood-derived M2 macrophages toward the M1 phenotype and increased secretion of M1-associated cytokines and chemokines. Moreover, the blockade of TIGIT augmented the anti-CD47-mediated phagocytosis of AML cell lines and primary AML cells.
Conclusion: Our findings suggest that immunosuppressive TIGIT+ M2 LAMs can be redirected into an efficient effector population that may be of direct clinical relevance in the near future.
Keywords: Costimulatory and Inhibitory T-Cell Receptors; Hematologic Neoplasms; Macrophages; Metabolic Networks and Pathways; Tumor Escape.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: FB: travel grant from Daiichi Sankyo, Servier, and Novartis; advisory board by Jazz, GmbH, Daiichi Sankyo. WF: personal fees and non-financial support from AbbVie; grants, personal fees, and non-financial support from Amgen and Pfizer; and personal fees from Jazz Pharmaceuticals, Celgene, Morphosys, Ariad/Incyte, Stemline Therapeutics, Daiichi Sankyo, and Servier outside the submitted work; in addition, WF has a patent for Amgen issued; and support for medical writing: Amgen, Pfizer, and AbbVie. The remaining authors declare that they have no conflict of interest.
Figures
References
-
- Sallman DA, Asch AS, Kambhampati S. The first-in-class anti-CD47 antibody magrolimab combined with azacitidine is well-tolerated and effective in AML patients: phase 1B results.. 2020 ASH Annu Meet Expo 2020 ASH Annu Meet Expo Abstr 330 Present December 6, 2020;2020:64–6.
-
- Tahk S, Schmitt S, Augsberger CP, et al. . Evaluation of a bifunctional Sirpα-CD123 fusion antibody for the elimination of acute myeloid leukemia stem cells. Blood 2019;134:2544. 10.1182/blood-2019-128145 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
