Influenza A and Respiratory Syncytial Virus Trigger a Cellular Response That Blocks Severe Acute Respiratory Syndrome Virus 2 Infection in the Respiratory Tract
- PMID: 36550077
- PMCID: PMC10266949
- DOI: 10.1093/infdis/jiac494
Influenza A and Respiratory Syncytial Virus Trigger a Cellular Response That Blocks Severe Acute Respiratory Syndrome Virus 2 Infection in the Respiratory Tract
Abstract
Background: Multiple viruses cocirculate and contribute to the burden of respiratory disease. Virus-virus interactions can decrease susceptibility to infection and this interference can have an epidemiological impact. As humans are normally exposed to a community of cocirculating respiratory viruses, experimental coinfection studies are necessary to understand the disease mechanisms of multipathogen systems. We aimed to characterize interactions within the respiratory tract between severe acute respiratory syndrome virus 2 (SARS-CoV-2) and 2 major respiratory viruses: influenza A virus (IAV), and respiratory syncytial virus (RSV).
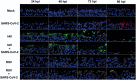
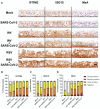
Methods: We performed single infections and coinfections with SARS-CoV-2 combined with IAV or RSV in cultures of human bronchial epithelial cells. We combined microscopy with quantification of viral replication in the presence or absence of an innate immune inhibitor to determine changes in virus-induced pathology, virus spread, and virus replication.
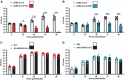
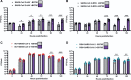
Results: SARS-CoV-2 replication is inhibited by both IAV and RSV. This inhibition is dependent on a functional antiviral response and the level of inhibition is proportional to the timing of secondary viral infection.
Conclusions: Infections with other respiratory viruses might provide transient resistance to SARS-CoV-2. It would therefore be expected that the incidence of coronavirus disease 2019 (COVID-19) may decrease during periods of high circulation of IAV and RSV.
Keywords: human airway epithelium; influenza A virus; respiratory syncytial virus; severe acute respiratory syndrome virus 2; virus coinfection.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Similar articles
-
Respiratory Syncytial Virus Protects Bystander Cells against Influenza A Virus Infection by Triggering Secretion of Type I and Type III Interferons.J Virol. 2022 Nov 23;96(22):e0134122. doi: 10.1128/jvi.01341-22. Epub 2022 Nov 3. J Virol. 2022. PMID: 36326278 Free PMC article.
-
Influenza A virus interferes with respiratory syncytial virus in mice and reconstituted human airway epithelium.Microbiol Spectr. 2025 Jun 3;13(6):e0318724. doi: 10.1128/spectrum.03187-24. Epub 2025 May 14. Microbiol Spectr. 2025. PMID: 40366152 Free PMC article.
-
Viral Interference During Influenza A-SARS-CoV-2 Coinfection of the Human Airway Epithelium and Reversal by Oseltamivir.J Infect Dis. 2024 May 15;229(5):1430-1434. doi: 10.1093/infdis/jiad402. J Infect Dis. 2024. PMID: 37722683 Free PMC article.
-
Assessing respiratory viral exclusion and affinity interactions through co-infection incidence in a pediatric population during the 2022 resurgence of influenza and RSV.Front Cell Infect Microbiol. 2023 Jun 14;13:1208235. doi: 10.3389/fcimb.2023.1208235. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37389220 Free PMC article. Review.
-
The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: A systematic review and meta-analysis.J Glob Health. 2022 Sep 17;12:05040. doi: 10.7189/jogh.12.05040. J Glob Health. 2022. PMID: 36112521 Free PMC article.
Cited by
-
Infection Rates and Symptomatic Proportion of SARS-CoV-2 and Influenza in Pediatric Population, China, 2023.Emerg Infect Dis. 2024 Sep;30(9):1809-1818. doi: 10.3201/eid3009.240065. Epub 2024 Aug 6. Emerg Infect Dis. 2024. PMID: 39106459 Free PMC article.
-
Viral interference between severe acute respiratory syndrome coronavirus 2 and influenza A viruses.PLoS Pathog. 2024 Jul 22;20(7):e1012017. doi: 10.1371/journal.ppat.1012017. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39038029 Free PMC article.
-
High Prevalence of Respiratory Co-Infections and Risk Factors in COVID-19 Patients at Hospital Admission During an Epidemic Peak in China.Infect Drug Resist. 2023 Oct 25;16:6781-6793. doi: 10.2147/IDR.S435143. eCollection 2023. Infect Drug Resist. 2023. PMID: 37904830 Free PMC article.
-
The inflammatory microenvironment of the lung at the time of infection governs innate control of SARS-CoV-2 replication.bioRxiv [Preprint]. 2024 Mar 27:2024.03.27.586885. doi: 10.1101/2024.03.27.586885. bioRxiv. 2024. Update in: Sci Immunol. 2024 Dec 6;9(102):eadp7951. doi: 10.1126/sciimmunol.adp7951. PMID: 38585846 Free PMC article. Updated. Preprint.
-
Reshaping Our Knowledge: Advancements in Understanding the Immune Response to Human Respiratory Syncytial Virus.Pathogens. 2023 Sep 1;12(9):1118. doi: 10.3390/pathogens12091118. Pathogens. 2023. PMID: 37764926 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

