Astrocytes amplify neurovascular coupling to sustained activation of neocortex in awake mice
- PMID: 36550102
- PMCID: PMC9780254
- DOI: 10.1038/s41467-022-35383-2
Astrocytes amplify neurovascular coupling to sustained activation of neocortex in awake mice
Abstract
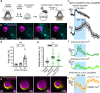
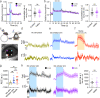
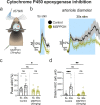
Functional hyperemia occurs when enhanced neuronal activity signals to increase local cerebral blood flow (CBF) to satisfy regional energy demand. Ca2+ elevation in astrocytes can drive arteriole dilation to increase CBF, yet affirmative evidence for the necessity of astrocytes in functional hyperemia in vivo is lacking. In awake mice, we discovered that functional hyperemia is bimodal with a distinct early and late component whereby arteriole dilation progresses as sensory stimulation is sustained. Clamping astrocyte Ca2+ signaling in vivo by expressing a plasma membrane Ca2+ ATPase (CalEx) reduces sustained but not brief sensory-evoked arteriole dilation. Elevating astrocyte free Ca2+ using chemogenetics selectively augments sustained hyperemia. Antagonizing NMDA-receptors or epoxyeicosatrienoic acid production reduces only the late component of functional hyperemia, leaving brief increases in CBF to sensory stimulation intact. We propose that a fundamental role of astrocyte Ca2+ is to amplify functional hyperemia when neuronal activation is prolonged.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

