The impact of increasing levels of blood C-reactive protein on the inflammatory loci SPI1 and CD33 in Alzheimer's disease
- PMID: 36550123
- PMCID: PMC9780312
- DOI: 10.1038/s41398-022-02281-6
The impact of increasing levels of blood C-reactive protein on the inflammatory loci SPI1 and CD33 in Alzheimer's disease
Abstract
Apolipoprotein ε4 (APOE ε4) is the most significant genetic risk factor for late-onset Alzheimer's disease (AD). Elevated blood C-reactive protein (CRP) further increases the risk of AD for people carrying the APOE ε4 allele. We hypothesized that CRP, as a key inflammatory element, could modulate the impact of other genetic variants on AD risk. We selected ten single nucleotide polymorphisms (SNPs) in reported AD risk loci encoding proteins related to inflammation. We then tested the interaction effects between these SNPs and blood CRP levels on AD incidence using the Cox proportional hazards model in UK Biobank (n = 279,176 white participants with 803 incident AD cases). The five top SNPs were tested for their interaction with different CRP cutoffs for AD incidence in the Framingham Heart Study (FHS) Generation 2 cohort (n = 3009, incident AD = 156). We found that for higher concentrations of serum CRP, the AD risk increased for SNP genotypes in 3 AD-associated genes (SPI1, CD33, and CLU). Using the Cox model in stratified genotype analysis, the hazard ratios (HRs) for the association between a higher CRP level (≥10 vs. <10 mg/L) and the risk of incident AD were 1.94 (95% CI: 1.33-2.84, p < 0.001) for the SPI1 rs1057233-AA genotype, 1.75 (95% CI: 1.20-2.55, p = 0.004) for the CD33 rs3865444-CC genotype, and 1.76 (95% CI: 1.25-2.48, p = 0.001) for the CLU rs9331896-C genotype. In contrast, these associations were not observed in the other genotypes of these genes. Finally, two SNPs were validated in 321 Alzheimer's Disease Neuroimaging (ADNI) Mild Cognitive Impairment (MCI) patients. We observed that the SPI1 and CD33 genotype effects were enhanced by elevated CRP levels for the risk of MCI to AD conversion. Furthermore, the SPI1 genotype was associated with CSF AD biomarkers, including t-Tau and p-Tau, in the ADNI cohort when the blood CRP level was increased (p < 0.01). Our findings suggest that elevated blood CRP, as a peripheral inflammatory biomarker, is an important moderator of the genetic effects of SPI1 and CD33 in addition to APOE ε4 on AD risk. Monitoring peripheral CRP levels may be helpful for precise intervention and prevention of AD for these genotype carriers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
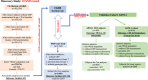
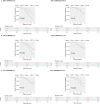


Similar articles
-
Association of CD33 rs3865444:C˃A polymorphism with a reduced risk of late-onset Alzheimer's disease in Slovaks is limited to subjects carrying the APOE ε4 allele.Int J Immunogenet. 2020 Oct;47(5):397-405. doi: 10.1111/iji.12489. Epub 2020 Apr 24. Int J Immunogenet. 2020. PMID: 32333488
-
Genome-wide association reveals genetic effects on human Aβ42 and τ protein levels in cerebrospinal fluids: a case control study.BMC Neurol. 2010 Oct 8;10:90. doi: 10.1186/1471-2377-10-90. BMC Neurol. 2010. PMID: 20932310 Free PMC article.
-
Associations of circulating C-reactive proteins, APOE ε4, and brain markers for Alzheimer's disease in healthy samples across the lifespan.Brain Behav Immun. 2022 Feb;100:243-253. doi: 10.1016/j.bbi.2021.12.008. Epub 2021 Dec 14. Brain Behav Immun. 2022. PMID: 34920091
-
Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease.JAMA Psychiatry. 2014 Oct;71(10):1183-91. doi: 10.1001/jamapsychiatry.2014.1060. JAMA Psychiatry. 2014. PMID: 25162367
-
Identification of novel quantitative traits-associated susceptibility loci for APOE ε 4 non-carriers of Alzheimer's disease.Curr Alzheimer Res. 2015;12(3):218-27. doi: 10.2174/1567205012666150302160145. Curr Alzheimer Res. 2015. PMID: 25731621
Cited by
-
Associations of Circulating Biomarkers with Disease Risks: a Two-Sample Mendelian Randomization Study.medRxiv [Preprint]. 2024 Jul 1:2024.06.30.24309729. doi: 10.1101/2024.06.30.24309729. medRxiv. 2024. Update in: Int J Mol Sci. 2024 Jul 05;25(13):7376. doi: 10.3390/ijms25137376. PMID: 39006413 Free PMC article. Updated. Preprint.
-
Associations of Circulating Biomarkers with Disease Risks: A Two-Sample Mendelian Randomization Study.Int J Mol Sci. 2024 Jul 5;25(13):7376. doi: 10.3390/ijms25137376. Int J Mol Sci. 2024. PMID: 39000484 Free PMC article.
-
Assessing quality of life and readmission rates among women in psychiatric care: a mixed-method study.BMC Psychol. 2025 Apr 10;13(1):361. doi: 10.1186/s40359-025-02687-z. BMC Psychol. 2025. PMID: 40211425 Free PMC article.
-
Cross-Sectional and Longitudinal Associations of Neighborhood Disadvantage With Fluid Biomarkers of Neuroinflammation and Neurodegeneration.Neurology. 2025 Jul 22;105(2):e213770. doi: 10.1212/WNL.0000000000213770. Epub 2025 Jun 25. Neurology. 2025. PMID: 40561381 Free PMC article.
-
Is Drp1 a link between mitochondrial dysfunction and inflammation in Alzheimer's disease?Front Mol Neurosci. 2023 May 12;16:1166879. doi: 10.3389/fnmol.2023.1166879. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37251647 Free PMC article. Review.
References
-
- Alzheimer’s disease. BMJ. 2009;338:b1349. 10.1136/bmj.b1349
-
- 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020; 10.1002/alz.12068
-
- Breteler MM, Bots ML, Ott A, Hofman A. Risk factors for vascular disease and dementia. Haemostasis. 1998;28:167–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

