Effects of phytoplankton, viral communities, and warming on free-living and particle-associated marine prokaryotic community structure
- PMID: 36550140
- PMCID: PMC9780322
- DOI: 10.1038/s41467-022-35551-4
Effects of phytoplankton, viral communities, and warming on free-living and particle-associated marine prokaryotic community structure
Abstract
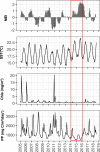
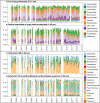
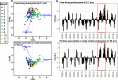
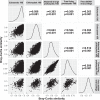
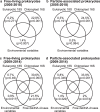
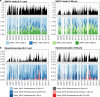
Free-living and particle-associated marine prokaryotes have physiological, genomic, and phylogenetic differences, yet factors influencing their temporal dynamics remain poorly constrained. In this study, we quantify the entire microbial community composition monthly over several years, including viruses, prokaryotes, phytoplankton, and total protists, from the San-Pedro Ocean Time-series using ribosomal RNA sequencing and viral metagenomics. Canonical analyses show that in addition to physicochemical factors, the double-stranded DNA viral community is the strongest factor predicting free-living prokaryotes, explaining 28% of variability, whereas the phytoplankton (via chloroplast 16S rRNA) community is strongest with particle-associated prokaryotes, explaining 31% of variability. Unexpectedly, protist community explains little variability. Our findings suggest that biotic interactions are significant determinants of the temporal dynamics of prokaryotes, and the relative importance of specific interactions varies depending on lifestyles. Also, warming influenced the prokaryotic community, which largely remained oligotrophic summer-like throughout 2014-15, with cyanobacterial populations shifting from cold-water ecotypes to warm-water ecotypes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Azam, F. et al. The ecological role of water-column microbes in the sea. Marine Ecol. Prog. Ser.10, 257–263 (1983).
-
- Fuhrman, J. A. & Caron D. A. in Manual of Environmental Microbiology (eds Yates, M. V. et al.) 4.2.2–4.2.2.-34 (ASM Press, 2016).
-
- Gasol, J. M. & Kirchman, D. L. Microbial Ecology of the Oceans (John Wiley & Sons, 2018).
-
- Gilbert JA, et al. The seasonal structure of microbial communities in the Western English Channel. Environ. Microbiol. 2009;11:3132–3139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

