Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature
- PMID: 36550156
- PMCID: PMC9780304
- DOI: 10.1038/s41467-022-35533-6
Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature
Abstract
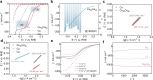
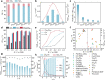
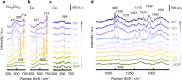
The development of electrocatalysts capable of efficient reduction of nitrate (NO3-) to ammonia (NH3) is drawing increasing interest for the sake of low carbon emission and environmental protection. Herein, we present a CuCo bimetallic catalyst able to imitate the bifunctional nature of copper-type nitrite reductase, which could easily remove NO2- via the collaboration of two active centers. Indeed, Co acts as an electron/proton donating center, while Cu facilitates NOx- adsorption/association. The bio-inspired CuCo nanosheet electrocatalyst delivers a 100 ± 1% Faradaic efficiency at an ampere-level current density of 1035 mA cm-2 at -0.2 V vs. Reversible Hydrogen Electrode. The NH3 production rate reaches a high activity of 4.8 mmol cm-2 h-1 (960 mmol gcat-1 h-1). A mechanistic study, using electrochemical in situ Fourier transform infrared spectroscopy and shell-isolated nanoparticle enhanced Raman spectroscopy, reveals a strong synergy between Cu and Co, with Co sites promoting the hydrogenation of NO3- to NH3 via adsorbed *H species. The well-modulated coverage of adsorbed *H and *NO3 led simultaneously to high NH3 selectivity and yield.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Enhancing Electrochemical Nitrate Reduction to Ammonia over Cu Nanosheets via Facet Tandem Catalysis.Angew Chem Int Ed Engl. 2023 Jun 26;62(26):e202303327. doi: 10.1002/anie.202303327. Epub 2023 May 15. Angew Chem Int Ed Engl. 2023. PMID: 37119055
-
Tungsten Nitride/Tungsten Oxide Nanosheets for Enhanced Oxynitride Intermediate Adsorption and Hydrogenation in Nitrate Electroreduction to Ammonia.ACS Nano. 2023 Dec 26;17(24):25091-25100. doi: 10.1021/acsnano.3c07734. Epub 2023 Dec 6. ACS Nano. 2023. PMID: 38054420
-
Synergistic CuCo tandem catalysis and F doping induced hydrogen bonding enable highly efficient nitrate reduction to ammonia.J Colloid Interface Sci. 2025 Aug 27;702(Pt 1):138838. doi: 10.1016/j.jcis.2025.138838. Online ahead of print. J Colloid Interface Sci. 2025. PMID: 40884885
-
Tuning Intermediates Adsorption and C─N Coupling for Efficient Urea Electrosynthesis Via Doping Ni into Cu.Small Methods. 2024 Mar;8(3):e2300811. doi: 10.1002/smtd.202300811. Epub 2023 Nov 23. Small Methods. 2024. PMID: 37997184 Review.
-
Recent Progress in the Electrochemical Formation of C-N Bonds for Construction of Organic Compounds via the Use of NOx/NOx.ChemSusChem. 2025 Feb 16;18(4):e202401751. doi: 10.1002/cssc.202401751. Epub 2024 Nov 9. ChemSusChem. 2025. PMID: 39375153 Review.
Cited by
-
In situ evolution of electrocatalysts for enhanced electrochemical nitrate reduction under realistic conditions.Environ Sci Ecotechnol. 2024 Sep 13;23:100492. doi: 10.1016/j.ese.2024.100492. eCollection 2025 Jan. Environ Sci Ecotechnol. 2024. PMID: 39398413 Free PMC article.
-
Integrating few-atom layer metal on high-entropy alloys to catalyze nitrate reduction in tandem.Nat Commun. 2024 Oct 18;15(1):9020. doi: 10.1038/s41467-024-53427-7. Nat Commun. 2024. PMID: 39424628 Free PMC article.
-
Leveraging Interfacial Electric Field for Smart Modulation of Electrode Surface in Nitrate to Ammonia Conversion.Adv Sci (Weinh). 2025 Jan;12(4):e2410763. doi: 10.1002/advs.202410763. Epub 2024 Dec 2. Adv Sci (Weinh). 2025. PMID: 39621532 Free PMC article.
-
Revealing and modulating catalyst reconstruction for highly efficient electrosynthesis of ammonia.Nat Commun. 2025 Jul 4;16(1):6161. doi: 10.1038/s41467-025-61075-8. Nat Commun. 2025. PMID: 40615397 Free PMC article.
-
Accelerating multielectron reduction at CuxO nanograins interfaces with controlled local electric field.Nat Commun. 2023 Nov 15;14(1):7383. doi: 10.1038/s41467-023-43303-1. Nat Commun. 2023. PMID: 37968299 Free PMC article.
References
-
- Ye S, et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 2022;15:760–770. doi: 10.1039/D1EE03097C. - DOI
-
- Nishina, K. New ammonia demand: ammonia fuel as a decarbonization tool and a new source of reactive nitrogen. Environ. Res. Lett. 17, 021003 (2022).
-
- Liu X, Elgowainy A, Wang M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green. Chem. 2020;22:5751–5761. doi: 10.1039/D0GC02301A. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

