The sky compass network in the brain of the desert locust
- PMID: 36550368
- PMCID: PMC10354188
- DOI: 10.1007/s00359-022-01601-x
The sky compass network in the brain of the desert locust
Abstract
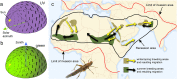
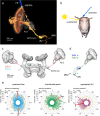
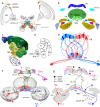
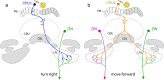
Many arthropods and vertebrates use celestial signals such as the position of the sun during the day or stars at night as compass cues for spatial orientation. The neural network underlying sky compass coding in the brain has been studied in great detail in the desert locust Schistocerca gregaria. These insects perform long-range migrations in Northern Africa and the Middle East following seasonal changes in rainfall. Highly specialized photoreceptors in a dorsal rim area of their compound eyes are sensitive to the polarization of the sky, generated by scattered sunlight. These signals are combined with direct information on the sun position in the optic lobe and anterior optic tubercle and converge from both eyes in a midline crossing brain structure, the central complex. Here, head direction coding is achieved by a compass-like arrangement of columns signaling solar azimuth through a 360° range of space by combining direct brightness cues from the sun with polarization cues matching the polarization pattern of the sky. Other directional cues derived from wind direction and internal self-rotation input are likely integrated. Signals are transmitted as coherent steering commands to descending neurons for directional control of locomotion and flight.
Keywords: Central complex; Desert locust; Intracellular recordings; Polarization vision; Sky compass coding.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Baker PS, Gewecke M, Cooter RJ. Flight orientation of swarming Locusta migratoria. Physiol Entomol. 1984;9:247–252. doi: 10.1111/j.1365-3032.1984.tb00706.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

