Real-world patient-reported outcomes and physician satisfaction with poly (ADP-ribose) polymerase inhibitors versus chemotherapy in patients with germline BRCA1/2-mutated human epidermal growth factor receptor 2-negative advanced breast cancer from the United States, Europe, and Israel
- PMID: 36550413
- PMCID: PMC9773591
- DOI: 10.1186/s12885-022-10325-9
Real-world patient-reported outcomes and physician satisfaction with poly (ADP-ribose) polymerase inhibitors versus chemotherapy in patients with germline BRCA1/2-mutated human epidermal growth factor receptor 2-negative advanced breast cancer from the United States, Europe, and Israel
Abstract
Background: In clinical trials, poly (ADP-ribose) polymerase inhibitors (PARPi) versus chemotherapy resulted in significantly improved progression-free survival, manageable adverse event profiles, and favorable patient-reported outcomes (PROs) in patients with human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC) and germline BRCA1/2 mutations (gBRCA1/2mut). The objective of this study was to evaluate PROs and physician satisfaction with treatment in patients with gBRCA1/2mut HER2- ABC receiving PARPi or physician's choice of chemotherapy in a multi-country, real-world setting.
Methods: This retrospective analysis used data from the Adelphi Real World ABC Disease Specific Programmes in the United States, European Union, and Israel. PROs were assessed at a single timepoint using the EuroQol 5-Dimensions 5-Level (EQ-5D-5L) scale, Cancer Therapy Satisfaction Questionnaire (CTSQ), and European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30 (EORTC QLQ-C30) and the breast cancer-specific module (QLQ-BR23). Baseline PROs were not assessed. Physician satisfaction with treatment scores was dichotomized to a 0/1 variable (0 = very dissatisfied/dissatisfied/moderately satisfied; 1 = satisfied/very satisfied). Scores were compared using inverse-probability-weighted regression adjustment, controlling for multiple confounding factors.
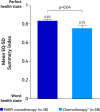
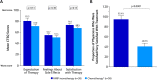
Results: The study included 96 patients (PARPi, n = 38; platinum/non-platinum-based chemotherapy, n = 58). Patients receiving PARPi versus chemotherapy reported significantly better scores on the EQ-5D-5L Health Utility Index. On the EORTC QLQ-C30 functional scales, patients receiving PARPi reported significantly better scores (mean ± SE) for physical functioning (80.0 ± 2.4 vs 71.9 ± 3.4; p < 0.05) and social functioning (82.0 ± 6.2 vs 63.6 ± 3.7; p < 0.05) and, on the symptom scales, reported significantly better scores for constipation (1.9 ± 1.8 vs 18.7 ± 3.2; p < 0.001), breast symptoms (0.4 ± 3.9 vs 13.3 ± 2.6; p < 0.01), arm symptoms (2.6 ± 1.3 vs 11.4 ± 2.4; p = 0.001), and systemic therapy side effects (13.5 ± 1.8 vs 29.4 ± 2.3; p < 0.001). In contrast, patients receiving chemotherapy scored significantly better on the nausea/vomiting scale (18.3 ± 2.8 vs 34.5 ± 5.1; p < 0.01). Patients receiving PARPi reported numerically better satisfaction scores on the CTSQ scales. Physicians were more likely to be satisfied/very satisfied with PARPi versus chemotherapy (95.4% ± 7.3% vs 40.8% ± 6.2%; p < 0.001).
Conclusions: The PRO findings in this real-world population of patients with gBRCA1/2mut HER2- ABC complement those from the pivotal clinical trials, providing further support for treatment with PARPi in these patients.
Keywords: Advanced breast cancer; BRCA1/2 mutation; Chemotherapy; Patient-reported outcomes; Poly (ADP-ribose) polymerase inhibitors; Quality of life; Real-world.
© 2022. Pfizer Inc.
Conflict of interest statement
RM has acted as consultant for Agendia, Amgen, AstraZeneca, Daiichi-Sankyo, Eisai, Genentech, Gilead, Hologic, Eli Lilly, Merck, Novartis, Pfizer, Puma, Sanofi, and SeaGen; and has contracted research for AstraZeneca, Genentech, and Veru.
AN, BA are employees of and own stock in Pfizer Inc.
KL, AR, and LM are employees of Adelphi Real World.
MPL has received honoraria for lectures, consulting, and/or working in an advisory role for Eli Lilly, AstraZeneca, MSD, Novartis, Pfizer, Eisai, Exact Sciences, Daiichi-Sankyo, Grünenthal, Gilead, Pierre Fabre, PharmaMar, Onkowissen, and Roche; received fees for travel, accommodations, and/or expenses from Roche and Pfizer; acted as editorial board member of Medac; and received fees for non-CME services from Eli Lilly, Roche, MSD, Novartis, Pfizer, Exact Sciences, Daiichi-Sankyo, Gilead, Grünenthal, AstraZeneca, and Eisai.
Figures

References
-
- US Food and Drug Administration. FDA approves olaparib for germline BRCA-mutated metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro.... Accessed 31 Jan 2022.
-
- US Food and Drug Administration. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-tala.... Accessed 31 Jan 2022.
-
- Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, Masuda N, Delaloge S, Li W, Armstrong A, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

