Synthesis, characterization, and anticancer evaluation of 1,3-bistetrahydrofuran-2yl-5-FU as a potential agent for pancreatic cancer
- PMID: 36550419
- PMCID: PMC9773620
- DOI: 10.1186/s12885-022-10449-y
Synthesis, characterization, and anticancer evaluation of 1,3-bistetrahydrofuran-2yl-5-FU as a potential agent for pancreatic cancer
Abstract
The failure of current chemotherapeutic agents for pancreatic cancer (PCa) makes it the most aggressive soft tissue tumor with a 5-year survival of slightly above 10% and is estimated to be the second leading cause of cancer death by 2030.
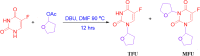
Objective: The main aim was to synthesize, characterize and evaluate the anticancer activity of 1,3-bistetrahydrofuran-2yl-5FU (MFU).
Methods: MFU was synthesized by using 5-fluorouracil (5-FU) and tetrahydrofuran acetate, and characterized by nuclear magnetic resonance (NMR), micro-elemental analysis, high-performance liquid chromatography (HPLC), and liquid chromatography with mass spectrophotometry (LC-MS). MFU and Gemcitabine hydrochloride (GemHCl) were tested for antiproliferative activity against MiaPaca-2 and Panc-1 cell lines.
Results: The half-minimum inhibitory concentration (IC50) of MFU was twice lower than that of GemHCl when used in both cell lines. MiaPaca-2 cells (MFU-IC50 = 4.5 ± 1.2 μM vs. GemHCl-IC50 = 10.3 ± 1.1 μM); meanwhile similar trend was observed in Panc-1 cells (MFU-IC50 = 3.0 ± 1 μM vs. GemHCl-IC50 = 6.1 ± 1.03 μM). The MFU and GemHCl effects on 3D spheroids showed a similar trend (IC50-GemHCl = 14.3 ± 1.1 μM vs. IC50-MFU = 7.2 ± 1.1 μM) for MiaPaca-2 cells, and (IC50-GemHCl = 16.3 ± 1.1 μM vs. IC50-MFU = 9.2 ± 1.1 μM) for Panc-1 cells. MFU significantly inhibited clonogenic cell growth, and induced cell death via apoptosis. Cell cycle data showed mean PI for GemHCl (48.5-55.7) twice higher than MFU (24.7 to 27.9) for MiaPaca-2 cells, and similarly to Panc-1 cells. The in-vivo model showed intensely stained EGFR (stained brown) in all control, GemHCl and MFU-treated mice bearing subcutaneous PDX tumors, however, HER2 expression was less stained in MFU-treated tumors compared to GemHCl-treated tumors and controls. Mean tumor volume of MFU-treated mice (361 ± 33.5 mm3) was three-fold lower than GemHCl-treated mice (1074 ± 181.2 mm3) bearing pancreatic PDX tumors.
Conclusion: MFU was synthesized with high purity and may have potential anticancer activity against PCa.
Keywords: Apoptosis; Cytotoxicity; Immunohistochemistry; Modified fluorouracil; Pancreatic cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ferlay J, et al. Global cancer observatory: cancer today. Lyon: International Agency for Research on Cancer; 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

