Non-interventional follow-up versus fluid bolus in RESPONSE to oliguria in hemodynamically stable critically ill patients: a randomized controlled pilot trial
- PMID: 36550559
- PMCID: PMC9773608
- DOI: 10.1186/s13054-022-04283-8
Non-interventional follow-up versus fluid bolus in RESPONSE to oliguria in hemodynamically stable critically ill patients: a randomized controlled pilot trial
Abstract
Background: Fluid bolus therapy is a common intervention to improve urine output. Data concerning the effect of a fluid bolus on oliguria originate mainly from observational studies and remain controversial regarding the actual benefit of such therapy. We compared the effect of a follow-up approach without fluid bolus to a 500 mL fluid bolus on urine output in hemodynamically stable critically ill patients with oliguria at least for 2 h (urine output < 0.5 mL/kg/h) in randomized setting.
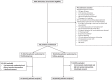
Methods: We randomized 130 patients in 1:1 fashion to receive either (1) non-interventional follow-up (FU) for 2 h or (2) 500 mL crystalloid fluid bolus (FB) administered over 30 min. The primary outcome was the proportion of patients who doubled their urine output, defined as 2-h urine output post-randomization divided by urine output 2 h pre-randomization. The outcomes were adjusted for the stratification variables (presence of sepsis or AKI) using two-tailed regression. Obtained odds ratios were converted to risk ratios (RR) with 95% confidence intervals (CI). The between-group difference in the continuous variables was compared using mean or median regression and expressed with 95% CIs.
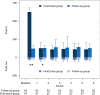
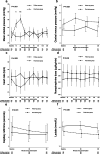
Results: Altogether 10 (15.9%) of 63 patients in the FU group and 22 (32.8%) of 67 patients in FB group doubled their urine output during the 2-h period, RR (95% CI) 0.49 (0.23-0.71), P = 0.026. Median [IQR] change in individual urine output 2 h post-randomization compared to 2 h pre-randomization was - 7 [- 19 to 17] mL in the FU group and 19[0-53] mL in the FB group, median difference (95% CI) - 23 (- 36 to - 10) mL, P = 0.001. Median [IQR] duration of oliguria in the FU group was 4 [2-8] h and in the FB group 2 [0-6] h, median difference (95%CI) 2 (0-4) h, P = 0.038. Median [IQR] cumulative fluid balance on study day was lower in the FU group compared to FB group, 678 [518-1029] mL versus 1071 [822-1505] mL, respectively, median difference (95%CI) - 387 (- 635 to - 213) mL, P < 0.001.
Conclusions: Follow-up approach to oliguria compared to administering a fluid bolus of 500 mL crystalloid in oliguric patients improved urine output less frequently but lead to lower cumulative fluid balance. Trial registration clinical.
Trials: gov, NCT02860572. Registered 9 August 2016.
Keywords: Acute kidney injury; Fluid balance; Fluid bolus; Oliguria; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

