Effect of Citrate- and Gold-Stabilized Superparamagnetic Iron Oxide Nanoparticles on Head and Neck Tumor Cell Lines during Combination Therapy with Ionizing Radiation
- PMID: 36551012
- PMCID: PMC9774466
- DOI: 10.3390/bioengineering9120806
Effect of Citrate- and Gold-Stabilized Superparamagnetic Iron Oxide Nanoparticles on Head and Neck Tumor Cell Lines during Combination Therapy with Ionizing Radiation
Abstract
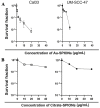
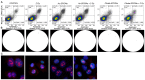
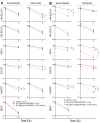
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide. They are associated with alcohol and tobacco consumption, as well as infection with human papillomaviruses (HPV). Therapeutic options include radiochemotherapy, surgery or chemotherapy. Nanoparticles are becoming more and more important in medicine. They can be used diagnostically, but also therapeutically. In order to provide therapeutic alternatives in the treatment of HNSCC, the effect of citrate-coated superparamagnetic iron oxide nanoparticles (Citrate-SPIONs) and gold-coated superparamagnetic iron oxide nanoparticles (Au-SPIONs) in combination with ionizing irradiation (IR) on two HPV positive and two HPV negative HNSCC and healthy fibroblasts and keratinocytes cell lines were tested. Effects on apoptosis and necrosis were analyzed by using flow cytometry. Cell survival studies were performed with a colony formation assay. To better understand where the SPIONs interact, light microscopy images and immunofluorescence studies were performed. The HNSCC and healthy cell lines showed different responses to the investigated SPIONs. The cytotoxic effects of SPIONs, in combination with IR, are dependent on the type of SPIONs, the dose administered and the cell type treated. They are independent of HPV status. Reasons for the different cytotoxic effect are probably the different compositions of the SPIONs and the related different interaction of the SPIONs intracellularly and paramembranously, which lead to different strong formations of double strand breaks.
Keywords: citrate; gold; head and neck cancer cell lines; interactions; ionizing radiation; nanoparticles; superparamagnetic iron oxide nanoparticles.
Conflict of interest statement
The other authors declare no conflict of interest.
Figures



Similar articles
-
In Vitro Analysis of Superparamagnetic Iron Oxide Nanoparticles Coated with APTES as Possible Radiosensitizers for HNSCC Cells.Nanomaterials (Basel). 2023 Jan 12;13(2):330. doi: 10.3390/nano13020330. Nanomaterials (Basel). 2023. PMID: 36678083 Free PMC article.
-
Synthesis and Characterization of Citrate-Stabilized Gold-Coated Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications.Molecules. 2020 Sep 26;25(19):4425. doi: 10.3390/molecules25194425. Molecules. 2020. PMID: 32993144 Free PMC article.
-
Superparamagnetic iron oxide nanoparticles induce persistent large foci of DNA damage in human melanoma cells post-irradiation.Radiat Environ Biophys. 2023 Aug;62(3):357-369. doi: 10.1007/s00411-023-01037-0. Epub 2023 Jul 15. Radiat Environ Biophys. 2023. PMID: 37452828
-
Toxicity assessment of superparamagnetic iron oxide nanoparticles in different tissues.Artif Cells Nanomed Biotechnol. 2020 Dec;48(1):443-451. doi: 10.1080/21691401.2019.1709855. Artif Cells Nanomed Biotechnol. 2020. PMID: 32024389 Review.
-
New Frontiers in Molecular Imaging with Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Efficacy, Toxicity, and Future Applications.Nucl Med Mol Imaging. 2020 Apr;54(2):65-80. doi: 10.1007/s13139-020-00635-w. Epub 2020 Feb 8. Nucl Med Mol Imaging. 2020. PMID: 32377258 Free PMC article. Review.
Cited by
-
In Vitro Analysis of Superparamagnetic Iron Oxide Nanoparticles Coated with APTES as Possible Radiosensitizers for HNSCC Cells.Nanomaterials (Basel). 2023 Jan 12;13(2):330. doi: 10.3390/nano13020330. Nanomaterials (Basel). 2023. PMID: 36678083 Free PMC article.
-
Therapeutic Approaches with Iron Oxide Nanoparticles to Induce Ferroptosis and Overcome Radioresistance in Cancers.Pharmaceuticals (Basel). 2025 Feb 26;18(3):325. doi: 10.3390/ph18030325. Pharmaceuticals (Basel). 2025. PMID: 40143107 Free PMC article. Review.
References
-
- Argiris A., Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat. Res. 2003;114:15–60. - PubMed
LinkOut - more resources
Full Text Sources

