Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies
- PMID: 36551014
- PMCID: PMC9774716
- DOI: 10.3390/bioengineering9120808
Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies
Abstract
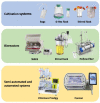
Harnessing the human immune system as a foundation for therapeutic technologies capable of recognizing and killing tumor cells has been the central objective of anti-cancer immunotherapy. In recent years, there has been an increasing interest in improving the effectiveness and accessibility of this technology to make it widely applicable for adoptive cell therapies (ACTs) such as chimeric antigen receptor T (CAR-T) cells, tumor infiltrating lymphocytes (TILs), dendritic cells (DCs), natural killer (NK) cells, and many other. Automated, scalable, cost-effective, and GMP-compliant bioreactors for production of ACTs are urgently needed. The primary efforts in the field of GMP bioreactors development are focused on closed and fully automated point-of-care (POC) systems. However, their clinical and industrial application has not yet reached full potential, as there are numerous obstacles associated with delicate balancing of the complex and often unpredictable cell biology with the need for precision and full process control. Here we provide a brief overview of the existing and most advanced systems for ACT manufacturing, including cell culture bags, G-Rex flasks, and bioreactors (rocking motion, stirred-flask, stirred-tank, hollow-fiber), as well as semi- and fully-automated closed bioreactor systems.
Keywords: CAR-T; T cell; adoptive cell immunotherapy; bioreactor; point-of-care.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zuliani T., David J., Bercegeay S., Pandolfino M.-C., Rodde-Astier I., Khammari A., Coissac C., Delorme B., Saïagh S., Dréno B. Value of Large Scale Expansion of Tumor Infiltrating Lymphocytes in a Compartmentalised Gas-Permeable Bag: Interests for Adoptive Immunotherapy. J. Transl. Med. 2011;9:63. doi: 10.1186/1479-5876-9-63. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

