A Hemin-Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment
- PMID: 36551037
- PMCID: PMC9776134
- DOI: 10.3390/bios12121070
A Hemin-Graphene Nanocomposite-Based Aptasensor for Ultrasensitive Colorimetric Quantification of Leukaemia Cells Using Magnetic Enrichment
Abstract
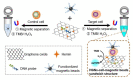
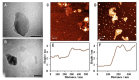
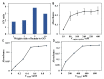
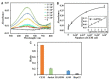
Diagnostic blood cell counting is of limited use in monitoring a minimal number of leukaemia cells, warranting further research to develop more sensitive and reliable techniques to identify leukaemia cells in circulation. In this work, a hemin-graphene nanocomposite-based aptasensor was developed for ultrasensitive colorimetric detection of leukaemia cells (CEM) using magnetic enrichment. Hemin-conjugated graphene oxide nanocomposites (HGNs) were prepared by hydrazine reduction using graphene oxide nanosheets and hemins. Hence, the prepared HGNs become able to absorb single-stranded DNA and acquire peroxidase-like activity. The aptamer sgc8c, which recognizes a specific target on leukaemia cells, was absorbed onto HGNs to capture the target CEM cancer cells. The captured target cells that associated with the HGNs were then concentrated and separated by magnetic beads (MBs) coated with sgc8c aptamers, forming a HGN-cell-MB sandwich structure. These sandwich structures can be quantified via an oxidation reaction catalysed by HGNs. By utilizing dual signal amplification effects generated by magnetic enrichment and the improved peroxidase activity of HGNs, the biosensor allowed for highly sensitive detection of 10 to 105 CEM cells with an ultra-low limit of detection (LOD) of 10 cells under optimal conditions. It is expected that the proposed aptasensor can be further employed in monitoring the minimal residual disease during the treatment of leukaemia.
Keywords: biosensors; graphene oxide; hemin; leukaemia cells; sgc8c aptamer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Glypican-3 electrochemical aptamer nanobiosensor based on hemin/graphene nanohybrids peroxidase-like catalytic silver deposition.Mikrochim Acta. 2020 Apr 30;187(5):305. doi: 10.1007/s00604-020-04284-w. Mikrochim Acta. 2020. PMID: 32356075
-
Fabrication of an ultrasensitive and selective electrochemical aptasensor to detect carcinoembryonic antigen by using a new nanocomposite.Biosens Bioelectron. 2019 Mar 15;129:1-6. doi: 10.1016/j.bios.2018.12.047. Epub 2019 Jan 3. Biosens Bioelectron. 2019. PMID: 30677696
-
An ATP Aptasensor Based on the Peroxidase-like Activity of Hemin/Graphene Oxide Nanosheets.Anal Sci. 2016;32(5):565-9. doi: 10.2116/analsci.32.565. Anal Sci. 2016. PMID: 27169657
-
Highly sensitive electrochemical aptasensor for Glypican-3 based on reduced graphene oxide-hemin nanocomposites modified on screen-printed electrode surface.Bioelectrochemistry. 2021 Apr;138:107696. doi: 10.1016/j.bioelechem.2020.107696. Epub 2020 Nov 17. Bioelectrochemistry. 2021. PMID: 33254049
-
Porphyrins as Colorimetric and Photometric Biosensors in Modern Bioanalytical Systems.Chembiochem. 2020 Jul 1;21(13):1793-1807. doi: 10.1002/cbic.202000067. Epub 2020 Mar 30. Chembiochem. 2020. PMID: 32187831 Free PMC article. Review.
Cited by
-
Aptamer-based approaches in leukemia: a paradigm shift in targeted therapy.Clin Exp Med. 2025 May 30;25(1):186. doi: 10.1007/s10238-025-01724-w. Clin Exp Med. 2025. PMID: 40445231 Free PMC article. Review.
-
In-Situ Fabrication of Electroactive Cu2+-Trithiocyanate Complex and Its Application for Label-Free Electrochemical Aptasensing of Thrombin.Biosensors (Basel). 2023 May 10;13(5):532. doi: 10.3390/bios13050532. Biosensors (Basel). 2023. PMID: 37232893 Free PMC article.
References
-
- Okikiolu J., Dillon R., Raj K. Acute leukaemia. Medicine. 2021;49:274–281. doi: 10.1016/j.mpmed.2021.02.004. - DOI
-
- Campos L., Guyotat D., Archimbaud E., Devaux Y., Treille D., Larese A., Maupas J., Gentilhomme O., Ehrsam A., Fiere D. Surface marker expression in adult acute myeloid leukaemia: Correlations with initial characteristics, morphology and response to therapy. Br. J. Haematol. 1989;72:161–166. doi: 10.1111/j.1365-2141.1989.tb07677.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

