Ir(III) Complexes with AIE Characteristics for Biological Applications
- PMID: 36551071
- PMCID: PMC9775350
- DOI: 10.3390/bios12121104
Ir(III) Complexes with AIE Characteristics for Biological Applications
Abstract
Both biological process detection and disease diagnosis on the basis of luminescence technology can provide comprehensive insights into the mechanisms of life and disease pathogenesis and also accurately guide therapeutics. As a family of prominent luminescent materials, Ir(III) complexes with aggregation-induced emission (AIE) tendency have been recently explored at a tremendous pace for biological applications, by virtue of their various distinct advantages, such as great stability in biological media, excellent fluorescence properties and distinctive photosensitizing features. Significant breakthroughs of AIE-active Ir(III) complexes have been achieved in the past few years and great progress has been witnessed in the construction of novel AIE-active Ir(III) complexes and their applications in organelle-specific targeting imaging, multiphoton imaging, biomarker-responsive bioimaging, as well as theranostics. This review systematically summarizes the basic concepts, seminal studies, recent trends and perspectives in this area.
Keywords: Ir(III) complexes; PDT; aggregation-induced emission (AIE); specific responsive; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


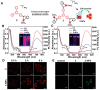

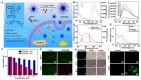




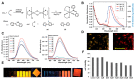

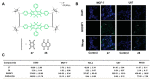




References
-
- Kober E.M., Meyer T.J. Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2′-bipyridine) Inorg. Chem. 1982;21:3967–3977. doi: 10.1021/ic00141a021. - DOI
-
- Flamigni L., Barbieri A., Sabatini C., Ventura B., Barigelletti F. Photochemistry and photophysics of coordination compounds: Iridium. Top. Curr. Chem. 2007;281:143–203. doi: 10.1007/128_2007_131. - DOI
-
- Alam P., Climent C., Alemany P., Laskar I.R. “Aggregation-induced emission” of transition metal compounds: Design, mechanistic insights, and applications. J. Photoch. Photobio. C Photoch. Rev. 2019;41 doi: 10.1016/j.jphotochemrev.2019.100317. - DOI
Publication types
MeSH terms
Grants and funding
- 52122317/National Natural Science Foundation of China
- 22175120/National Natural Science Foundation of China
- RCYX20200714114525101/Developmental Fund for Science and Technology of Shenzhen government
- JCYJ20190808153415062/Developmental Fund for Science and Technology of Shenzhen government
- 2020B1515020011/Natural Science Foundation for Distinguished Young Scholars of Guangdong Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

