Organic Light-Emitting Diode Based Fluorescence-Linked Immunosorbent Assay for SARS-CoV-2 Antibody Detection
- PMID: 36551092
- PMCID: PMC9775261
- DOI: 10.3390/bios12121125
Organic Light-Emitting Diode Based Fluorescence-Linked Immunosorbent Assay for SARS-CoV-2 Antibody Detection
Abstract
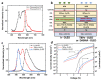
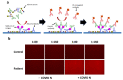
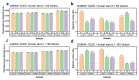
Immunodiagnostics have been widely used in the detection of disease biomarkers. The conventional immunological tests in central laboratories require expensive equipment and, for non-specialists, the tests are technically demanding and time-consuming, which has prevented their use by the public. Thus, point-of-care tests (POCT), such as lateral flow immunoassays, are being, or have been, developed as more convenient and low-cost methods for immunodiagnostics. However, the sensitivity of such tests is often a concern. Here, a fluorescence-linked immunosorbent assay (FLISA) using organic light-emitting diodes (OLEDs) as excitation light sources was investigated as a way forward for the development of compact and sensitive POCTs. Phycoerythrin (PE) was selected as the fluorescent dye, and OLEDs were designed with different emission spectra. The leakage light of different OLEDs for exciting PE was then investigated to reduce the background noise and improve the sensitivity of the system. Finally, as proof-of-principle that OLED-based technology can be successfully further developed for POCT, antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human serum was detected by OLED-FLISA.
Keywords: FLISA; OLED; organic semiconductor; point-of-care testing; sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Digital and Analog Detection of SARS-CoV-2 Nucleocapsid Protein via an Upconversion-Linked Immunosorbent Assay.Anal Chem. 2023 Mar 14;95(10):4753-4759. doi: 10.1021/acs.analchem.2c05670. Epub 2023 Feb 27. Anal Chem. 2023. PMID: 36916131 Free PMC article.
-
Establishment of an Immunological Method for Detection of Bluetongue Virus by Fluorescence-Linked Immunosorbent Assay.Microbiol Spectr. 2022 Oct 26;10(5):e0142922. doi: 10.1128/spectrum.01429-22. Epub 2022 Sep 26. Microbiol Spectr. 2022. PMID: 36154153 Free PMC article.
-
Integrated OLED as excitation light source in fluorescent lateral flow immunoassays.Biosens Bioelectron. 2015 Dec 15;74:150-5. doi: 10.1016/j.bios.2015.06.049. Epub 2015 Jun 20. Biosens Bioelectron. 2015. PMID: 26134292
-
Recent advances in quantum dot-based fluorescence-linked immunosorbent assays.Nanoscale. 2023 Mar 23;15(12):5560-5578. doi: 10.1039/d2nr07247e. Nanoscale. 2023. PMID: 36866747 Review.
-
Immunochromatographic enhancement strategy for SARS-CoV-2 detection based on nanotechnology.Nanoscale. 2023 Sep 29;15(37):15092-15107. doi: 10.1039/d3nr02396f. Nanoscale. 2023. PMID: 37676509 Review.
Cited by
-
The Influence of Thickness and Spectral Properties of Green Color-Emitting Polymer Thin Films on Their Implementation in Wearable PLED Applications.Nanomaterials (Basel). 2024 Oct 7;14(19):1608. doi: 10.3390/nano14191608. Nanomaterials (Basel). 2024. PMID: 39404335 Free PMC article.
-
Organic Electronics-Microfluidics/Lab on a Chip Integration in Analytical Applications.Sensors (Basel). 2023 Oct 16;23(20):8488. doi: 10.3390/s23208488. Sensors (Basel). 2023. PMID: 37896581 Free PMC article. Review.
-
Fluorescence Multi-Detection Device Using a Lensless Matrix Addressable microLED Array.Biosensors (Basel). 2024 May 22;14(6):264. doi: 10.3390/bios14060264. Biosensors (Basel). 2024. PMID: 38920568 Free PMC article.
References
-
- Bietenbeck A., Junker R., Luppa P.B. Central Laboratory Service and Point-of-Care Testing in Germany—From Conflicting Notions to Complementary Understandings. Point Care. 2015;14:1–11. doi: 10.1097/POC.0000000000000043. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

