Stabilization of Copper-Based Biochips with Alumina for Biosensing Application
- PMID: 36551099
- PMCID: PMC9775342
- DOI: 10.3390/bios12121132
Stabilization of Copper-Based Biochips with Alumina for Biosensing Application
Abstract
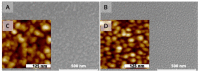
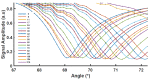
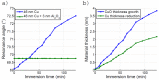
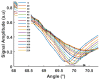
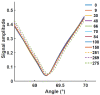
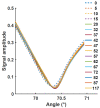
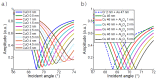
Surface plasmon resonance devices typically rely on the use of gold-coated surfaces, but the use of more abundant metals is desirable for the long-term development of plasmonic biochips. As a substitute for gold, thin copper films have been deposited on glass coverslips by thermal evaporation. As expected, these films immersed in a water solution initially exhibit an intense plasmonic resonance comparable to gold. However, without protection, an angle-resolved optical analysis shows a rapid degradation of the copper, characterized by a continuous angular shift of the plasmonic resonance curve. We show that copper films protected with a thin layer of aluminum oxide of a few nanometers can limit the oxidation rate for a sufficient time to perform some standard measurements. As the process is simple and compatible with the current biochip production technique, such an approach could pave the way for the production of alternative and more sustainable biochips.
Keywords: biochips; biosensors; copper; plasmonic; surface plasmon resonance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hagelüken C. Recycling of (critical) metals. Crit. Met. Handb. 2014:41–69. doi: 10.1002/9781118755341.ch3. - DOI
-
- Li L., Liu G., Pan D., Wang W., Wu Y., Zuo T. Overview of the recycling technology for copper-containing cables. Resour. Conserv. Recycl. 2017;126:132–140. doi: 10.1016/j.resconrec.2017.07.024. - DOI
-
- Figueiredo V., Elangovan E., Gonçalves G., Barquinha P., Pereira L., Franco N., Alves E., Martins R., Fortunato E. Effect of post-annealing on the properties of copper oxide thin films obtained from the oxidation of evaporated metallic copper. Appl. Surf. Sci. 2008;254:3949–3954. doi: 10.1016/j.apsusc.2007.12.019. - DOI
-
- Maeda Y., Akita T., Kohyama M. High Activity of Gold/Tin-Dioxide Catalysts for Low-Temperature CO Oxidation: Application of a Reducible Metal Oxide to a Catalyst Support. Catal. Lett. 2014;144:2086–2090. doi: 10.1007/s10562-014-1376-4. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

