Conducting Polymer-Infused Electrospun Fibre Mat Modified by POEGMA Brushes as Antifouling Biointerface
- PMID: 36551110
- PMCID: PMC9775683
- DOI: 10.3390/bios12121143
Conducting Polymer-Infused Electrospun Fibre Mat Modified by POEGMA Brushes as Antifouling Biointerface
Abstract
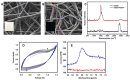
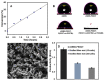
Biofouling on surfaces, caused by the assimilation of proteins, peptides, lipids and microorganisms, leads to contamination, deterioration and failure of biomedical devices and causes implants rejection. To address these issues, various antifouling strategies have been extensively studied, including polyethylene glycol-based polymer brushes. Conducting polymers-based biointerfaces have emerged as advanced surfaces for interfacing biological tissues and organs with electronics. Antifouling of such biointerfaces is a challenge. In this study, we fabricated electrospun fibre mats from sulphonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (sSEBS), infused with conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) (sSEBS-PEDOT), to produce a conductive (2.06 ± 0.1 S/cm), highly porous, fibre mat that can be used as a biointerface in bioelectronic applications. To afford antifouling, here the poly(oligo (ethylene glycol) methyl ether methacrylate) (POEGMA) brushes were grafted onto the sSEBS-PEDOT conducting fibre mats via surface-initiated atom transfer radical polymerization technique (SI-ATRP). For that, a copolymer of EDOT and an EDOT derivative with SI-ATRP initiating sites, 3,4-ethylenedioxythiophene) methyl 2-bromopropanoate (EDOTBr), was firstly electropolymerized on the sSEBS-PEDOT fibre mat to provide sSEBS-PEDOT/P(EDOT-co-EDOTBr). The POEGMA brushes were grafted from the sSEBS-PEDOT/P(EDOT-co-EDOTBr) and the polymerization kinetics confirmed the successful growth of the brushes. Fibre mats with 10-mers and 30-mers POEGMA brushes were studied for antifouling using a BCA protein assay. The mats with 30-mers grafted brushes exhibited excellent antifouling efficiency, ~82% of proteins repelled, compared to the pristine sSEBS-PEDOT fibre mat. The grafted fibre mats exhibited cell viability >80%, comparable to the standard cell culture plate controls. Such conducting, porous biointerfaces with POEGMA grafted brushes are suitable for applications in various biomedical devices, including biosensors, liquid biopsy, wound healing substrates and drug delivery systems.
Keywords: BSA; POEGMA brush; antifouling; cell viability and proliferation; conducting polymer; electrospinning; proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Grafting of Porous Conductive Fiber Mats with an Antifouling Polymer Brush by Means of Filtration-Based Surface Initiated ATRP.Macromol Rapid Commun. 2025 Apr;46(8):e2300069. doi: 10.1002/marc.202300069. Epub 2023 Apr 6. Macromol Rapid Commun. 2025. PMID: 36965049 Free PMC article.
-
Long-range interactions between protein-coated particles and POEGMA brush layers in a serum environment.Colloids Surf B Biointerfaces. 2017 Feb 1;150:279-287. doi: 10.1016/j.colsurfb.2016.10.040. Epub 2016 Oct 24. Colloids Surf B Biointerfaces. 2017. PMID: 28341156
-
Understanding the Oxidative Stability of Antifouling Polymer Brushes.Langmuir. 2017 Jul 25;33(29):7298-7304. doi: 10.1021/acs.langmuir.7b01681. Epub 2017 Jul 10. Langmuir. 2017. PMID: 28650665
-
Molecular level studies on interfacial hydration of zwitterionic and other antifouling polymers in situ.Acta Biomater. 2016 Aug;40:6-15. doi: 10.1016/j.actbio.2016.02.030. Epub 2016 Feb 23. Acta Biomater. 2016. PMID: 26923530 Review.
-
Surface Functionalization with Polymer Brushes via Surface-Initiated Atom Transfer Radical Polymerization: Synthesis, Applications, and Current Challenges.Langmuir. 2024 Mar 19;40(11):5571-5589. doi: 10.1021/acs.langmuir.3c03647. Epub 2024 Mar 5. Langmuir. 2024. PMID: 38440955 Review.
Cited by
-
Grafting of Porous Conductive Fiber Mats with an Antifouling Polymer Brush by Means of Filtration-Based Surface Initiated ATRP.Macromol Rapid Commun. 2025 Apr;46(8):e2300069. doi: 10.1002/marc.202300069. Epub 2023 Apr 6. Macromol Rapid Commun. 2025. PMID: 36965049 Free PMC article.
References
-
- Riedel T., Májek P., Riedelová-Reicheltová Z., Vorobii M., Houska M., Rodriguez-Emmenegger C. Total removal of intact blood plasma proteins deposited on surface-grafted polymer brushes. Anal. Methods. 2016;8:6415–6419. doi: 10.1039/C6AY01833E. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

