Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques
- PMID: 36551140
- PMCID: PMC9775119
- DOI: 10.3390/bios12121173
Assessing the Food Quality Using Carbon Nanomaterial Based Electrodes by Voltammetric Techniques
Abstract
The world is facing a global financial loss and health effects due to food quality adulteration and contamination, which are seriously affecting human health. Synthetic colors, flavors, and preservatives are added to make food more attractive to consumers. Therefore, food safety has become one of the fundamental needs of mankind. Due to the importance of food safety, the world is in great need of developing desirable and accurate methods for determining the quality of food. In recent years, the electrochemical methods have become more popular, due to their simplicity, ease in handling, economics, and specificity in determining food safety. Common food contaminants, such as pesticides, additives, and animal drug residues, cause foods that are most vulnerable to contamination to undergo evaluation frequently. The present review article discusses the electrochemical detection of the above food contaminants using different carbon nanomaterials, such as carbon nanotubes (CNTs), graphene, ordered mesoporous carbon (OMC), carbon dots, boron doped diamond (BDD), and fullerenes. The voltammetric methods, such as cyclic voltammetry (CV) and differential pulse voltammetry (DPV), have been proven to be potential methods for determining food contaminants. The use of carbon-based electrodes has the added advantage of electrochemically sensing the food contaminants due to their excellent sensitivity, specificity, large surface area, high porosity, antifouling, and biocompatibility.
Keywords: cyclic voltammetry; differential pulse voltammetry; electrochemical sensors; food safety; graphene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







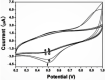
References
-
- World Health Organization Food Safety. [(accessed on 19 May 2022)];2022 Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety.
-
- Mahale R.S., Shashanka R., Shamanth V., Vinay Kumar R. Voltammetric Determination of Various Food Azo Dyes Using Different Modified Carbon Paste Electrodes. Biointerface Res. Appl. Chem. 2022;12:4557–4566.
-
- Haque M.A., Morozova K., Ferrentino G., Scampicchio M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis. 2021;33:1419–1435. doi: 10.1002/elan.202060600. - DOI
-
- Shashanka R., Chaira D., Swamy B.E.K. Electrocatalytic Response of Duplex and Yittria Dispersed Duplex Stainless Steel Modified Carbon Paste Electrode in Detecting Folic Acid Using Cyclic Voltammetry. Int. J. Electrochem. Sci. 2015;10:5586–5598.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

