Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins
- PMID: 36551154
- PMCID: PMC9775454
- DOI: 10.3390/biom12121726
Dual Role of ACBD6 in the Acylation Remodeling of Lipids and Proteins
Abstract
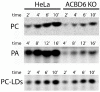
The transfer of acyl chains to proteins and lipids from acyl-CoA donor molecules is achieved by the actions of diverse enzymes and proteins, including the acyl-CoA binding domain-containing protein ACBD6. N-myristoyl-transferase (NMT) enzymes catalyze the covalent attachment of a 14-carbon acyl chain from the relatively rare myristoyl-CoA to the N-terminal glycine residue of myr-proteins. The interaction of the ankyrin-repeat domain of ACBD6 with NMT produces an active enzymatic complex for the use of myristoyl-CoA protected from competitive inhibition by acyl donor competitors. The absence of the ACBD6/NMT complex in ACBD6.KO cells increased the sensitivity of the cells to competitors and significantly reduced myristoylation of proteins. Protein palmitoylation was not altered in those cells. The specific defect in myristoyl-transferase activity of the ACBD6.KO cells provided further evidence of the essential functional role of the interaction of ACBD6 with the NMT enzymes. Acyl-CoAs bound to the acyl-CoA binding domain of ACBD6 are acyl donors for the lysophospholipid acyl-transferase enzymes (LPLAT), which acylate single acyl-chain lipids, such as the bioactive molecules LPA and LPC. Whereas the formation of acyl-CoAs was not altered in ACBD6.KO cells, lipid acylation processes were significantly reduced. The defect in PC formation from LPC by the LPCAT enzymes resulted in reduced lipid droplets content. The diversity of the processes affected by ACBD6 highlight its dual function as a carrier and a regulator of acyl-CoA dependent reactions. The unique role of ACBD6 represents an essential common feature of (acyl-CoA)-dependent modification pathways controlling the lipid and protein composition of human cell membranes.
Keywords: acylation; lipid droplets; lysophospholipids; myristoylation; palmitoylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Requirement of the acyl-CoA carrier ACBD6 in myristoylation of proteins: Activation by ligand binding and protein interaction.PLoS One. 2020 Feb 27;15(2):e0229718. doi: 10.1371/journal.pone.0229718. eCollection 2020. PLoS One. 2020. PMID: 32108178 Free PMC article.
-
Association of NMT2 with the acyl-CoA carrier ACBD6 protects the N-myristoyltransferase reaction from palmitoyl-CoA.J Lipid Res. 2016 Feb;57(2):288-98. doi: 10.1194/jlr.M065003. Epub 2015 Nov 30. J Lipid Res. 2016. PMID: 26621918 Free PMC article.
-
ACBD6 protein controls acyl chain availability and specificity of the N-myristoylation modification of proteins.J Lipid Res. 2019 Mar;60(3):624-635. doi: 10.1194/jlr.M091397. Epub 2019 Jan 14. J Lipid Res. 2019. PMID: 30642881 Free PMC article.
-
Compartmentalised acyl-CoA metabolism and roles in chromatin regulation.Mol Metab. 2020 Aug;38:100941. doi: 10.1016/j.molmet.2020.01.005. Epub 2020 Feb 14. Mol Metab. 2020. PMID: 32199817 Free PMC article. Review.
-
Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells.J Biochem. 1997 Jul;122(1):1-16. doi: 10.1093/oxfordjournals.jbchem.a021715. J Biochem. 1997. PMID: 9276665 Review.
Cited by
-
Candidate genes associated with fatty acid compositions in north American Atlantic salmon (Salmo salar).BMC Genomics. 2024 Dec 18;25(1):1208. doi: 10.1186/s12864-024-11131-2. BMC Genomics. 2024. PMID: 39695999 Free PMC article.
-
Identification and characterization of interacting proteins of transcription factor DpWRI1-like related to lipid biosynthesis from microalga Dunaliella parva.Heliyon. 2024 Dec 12;11(1):e41165. doi: 10.1016/j.heliyon.2024.e41165. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39758396 Free PMC article.
-
Identification of lipid metabolism-related gene signature in the bone marrow microenvironment of multiple myelomas through deep analysis of transcriptomic data.Clin Exp Med. 2024 Jun 25;24(1):136. doi: 10.1007/s10238-024-01398-w. Clin Exp Med. 2024. PMID: 38916672 Free PMC article.
-
Deciphering the role of lipid metabolism-related genes in Alzheimer's disease: a machine learning approach integrating Traditional Chinese Medicine.Front Endocrinol (Lausanne). 2024 Oct 23;15:1448119. doi: 10.3389/fendo.2024.1448119. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39507054 Free PMC article.
References
-
- Castrec B., Dian C., Ciccone S., Ebert C.L., Bienvenut W.V., Le Caer J.P., Steyaert J.M., Giglione C., Meinnel T. Structural and genomic decoding of human and plant myristoylomes reveals a definitive recognition pattern. Nat. Chem. Biol. 2018;14:671–679. doi: 10.1038/s41589-018-0077-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

