Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease
- PMID: 36551160
- PMCID: PMC9775496
- DOI: 10.3390/biom12121732
Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease
Abstract
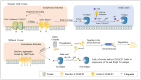
The core-1 β1-3galactosyltransferase-specific chaperone 1 (Cosmc) is a unique molecular chaperone of core-1 β1-3galactosyltransferase(C1GALT1), which typically functions inside the endoplasmic reticulum (ER). Cosmc helps C1GALT1 to fold correctly and maintain activity. It also participates in the synthesis of the T antigen, O-glycan, together with C1GALT1. Cosmc is a multifaceted molecule with a wide range of roles and functions. It involves platelet production and the regulation of immune cell function. Besides that, the loss of function of Cosmc also facilitates the development of several diseases, such as inflammation diseases, immune-mediated diseases, and cancer. It suggests that Cosmc is a critical control point in diseases and that it should be regarded as a potential target for oncotherapy. It is essential to fully comprehend Cosmc's roles, as they may provide critical information about its involvement in disease development and pathogenesis. In this review, we summarize the recent progress in understanding the role of Cosmc in normal development and diseases.
Keywords: C1GALT1; Cosmc; O-glycosylation; Tn antigen; chaperonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stotter B.R., Talbot B.E., Capen D.E., Artelt N., Zeng J., Matsumoto Y., Endlich N., Cummings R.D., Schlondorff J.S. Cosmc-dependent mucin-type O-linked glycosylation is essential for podocyte function. Am. J. Physiol. Renal. Physiol. 2020;318:F518–F530. doi: 10.1152/ajprenal.00399.2019. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

