Pyroptosis in Periprosthetic Osteolysis
- PMID: 36551161
- PMCID: PMC9775904
- DOI: 10.3390/biom12121733
Pyroptosis in Periprosthetic Osteolysis
Abstract
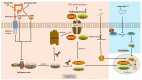
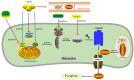
Periprosthetic osteolysis (PPO) along with aseptic loosening (AL) caused by wear particles after artificial joint replacement is the key factor in surgical failure and subsequent revision surgery, however, the precise molecular mechanism underlying PPO remains unclear. Aseptic inflammation triggered by metal particles, resulting in the imbalance between bone formation by osteoblasts and bone resorption by osteoclasts may be the decisive factor. Pyroptosis is a new pro-inflammatory pattern of regulated cell death (RCD), mainly mediated by gasdermins (GSDMs) family, among which GSDMD is the best characterized. Recent evidence indicates that activation of NLRP3 inflammasomes and pyroptosis play a pivotal role in the pathological process of PPO. Here, we review the pathological process of PPO, the molecular mechanism of pyroptosis and the interventions to inhibit the inflammation and pyroptosis of different cells during the PPO. Conclusively, this review provides theoretical support for the search for new strategies and new targets for the treatment of PPO by inhibiting pyroptosis and inflammation.
Keywords: NLRP3; aseptic loosening; inflammation; periprosthetic osteolysis; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis.Front Immunol. 2023 Oct 4;14:1274679. doi: 10.3389/fimmu.2023.1274679. eCollection 2023. Front Immunol. 2023. PMID: 37860014 Free PMC article. Review.
-
Blockade of XCL1/Lymphotactin Ameliorates Severity of Periprosthetic Osteolysis Triggered by Polyethylene-Particles.Front Immunol. 2020 Aug 4;11:1720. doi: 10.3389/fimmu.2020.01720. eCollection 2020. Front Immunol. 2020. PMID: 32849609 Free PMC article.
-
Apelin-13 alleviates osteoclast formation and osteolysis through Nrf2-pyroptosis pathway.Microsc Res Tech. 2024 Jun;87(6):1348-1358. doi: 10.1002/jemt.24519. Epub 2024 Feb 21. Microsc Res Tech. 2024. PMID: 38380581
-
Quercetin alleviates nanoparticle-induced osteolysis via deactivating pyroptosis.Biomater Sci. 2023 Jun 27;11(13):4616-4629. doi: 10.1039/d3bm00060e. Biomater Sci. 2023. PMID: 37199324
-
Roles of inflammatory cell infiltrate in periprosthetic osteolysis.Front Immunol. 2023 Dec 1;14:1310262. doi: 10.3389/fimmu.2023.1310262. eCollection 2023. Front Immunol. 2023. PMID: 38106424 Free PMC article. Review.
Cited by
-
Angiopoietin 1 Relieves Osteolysis by Promoting Macrophage Mitophagy Through the TBK1-SQSTM1 Pathway to Inhibit AIM2 Inflammasome-Mediated Pyroptosis.Appl Biochem Biotechnol. 2024 Nov;196(11):7908-7927. doi: 10.1007/s12010-024-04961-z. Epub 2024 Apr 25. Appl Biochem Biotechnol. 2024. PMID: 38662322
-
Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis.Front Immunol. 2023 Oct 4;14:1274679. doi: 10.3389/fimmu.2023.1274679. eCollection 2023. Front Immunol. 2023. PMID: 37860014 Free PMC article. Review.
-
ROS: Executioner of regulating cell death in spinal cord injury.Front Immunol. 2024 Jan 23;15:1330678. doi: 10.3389/fimmu.2024.1330678. eCollection 2024. Front Immunol. 2024. PMID: 38322262 Free PMC article. Review.
-
A mechanistic review of the pharmacological aspects of Kaempferide as a natural compound.Heliyon. 2024 Sep 24;10(19):e38243. doi: 10.1016/j.heliyon.2024.e38243. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39397988 Free PMC article. Review.
-
From death to birth: how osteocyte death promotes osteoclast formation.Front Immunol. 2025 Mar 17;16:1551542. doi: 10.3389/fimmu.2025.1551542. eCollection 2025. Front Immunol. 2025. PMID: 40165960 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

