Sequence-Based Prediction of Protein Phase Separation: The Role of Beta-Pairing Propensity
- PMID: 36551199
- PMCID: PMC9775558
- DOI: 10.3390/biom12121771
Sequence-Based Prediction of Protein Phase Separation: The Role of Beta-Pairing Propensity
Abstract
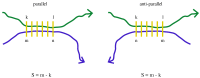
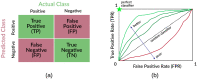
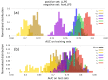
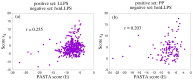
The formation of droplets of bio-molecular condensates through liquid-liquid phase separation (LLPS) of their component proteins is a key factor in the maintenance of cellular homeostasis. Different protein properties were shown to be important in LLPS onset, making it possible to develop predictors, which try to discriminate a positive set of proteins involved in LLPS against a negative set of proteins not involved in LLPS. On the other hand, the redundancy and multivalency of the interactions driving LLPS led to the suggestion that the large conformational entropy associated with non specific side-chain interactions is also a key factor in LLPS. In this work we build a LLPS predictor which combines the ability to form pi-pi interactions, with an unrelated feature, the propensity to stabilize the β-pairing interaction mode. The cross-β structure is formed in the amyloid aggregates, which are involved in degenerative diseases and may be the final thermodynamically stable state of protein condensates. Our results show that the combination of pi-pi and β-pairing propensity yields an improved performance. They also suggest that protein sequences are more likely to be involved in phase separation if the main chain conformational entropy of the β-pairing maintained droplet state is increased. This would stabilize the droplet state against the more ordered amyloid state. Interestingly, the entropic stabilization of the droplet state appears to proceed according to different mechanisms, depending on the fraction of "droplet-driving" proteins present in the positive set.
Keywords: amyloid aggregates; liquid-liquid phase separation; protein droplets.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Phase Separation of Epstein-Barr Virus EBNA2 and Its Coactivator EBNALP Controls Gene Expression.J Virol. 2020 Mar 17;94(7):e01771-19. doi: 10.1128/JVI.01771-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941785 Free PMC article.
-
Amyloid Aggregation and Liquid-Liquid Phase Separation from the Perspective of Phase Transitions.J Phys Chem B. 2023 Jul 20;127(28):6241-6250. doi: 10.1021/acs.jpcb.3c01426. Epub 2023 Jul 6. J Phys Chem B. 2023. PMID: 37414583 Free PMC article. Review.
-
Mechanism underlying liquid-to-solid phase transition in fused in sarcoma liquid droplets.Phys Chem Chem Phys. 2022 Aug 17;24(32):19346-19353. doi: 10.1039/d2cp02171d. Phys Chem Chem Phys. 2022. PMID: 35943083
-
Watching liquid droplets of TDP-43CTD age by Raman spectroscopy.J Biol Chem. 2022 Feb;298(2):101528. doi: 10.1016/j.jbc.2021.101528. Epub 2021 Dec 23. J Biol Chem. 2022. PMID: 34953857 Free PMC article.
-
Raman spectroscopy and imaging of protein droplet formation and aggregation.Curr Opin Struct Biol. 2025 Jun;92:103041. doi: 10.1016/j.sbi.2025.103041. Epub 2025 Apr 1. Curr Opin Struct Biol. 2025. PMID: 40174314 Review.
Cited by
-
Stability of Protein Pharmaceuticals: Recent Advances.Pharm Res. 2024 Jul;41(7):1301-1367. doi: 10.1007/s11095-024-03726-x. Epub 2024 Jun 27. Pharm Res. 2024. PMID: 38937372 Review.
-
Location and Concentration of Aromatic-Rich Segments Dictates the Percolating Inter-Molecular Network and Viscoelastic Properties of Ageing Condensates.Adv Sci (Weinh). 2023 Sep;10(25):e2207742. doi: 10.1002/advs.202207742. Epub 2023 Jun 29. Adv Sci (Weinh). 2023. PMID: 37386790 Free PMC article.
-
Membraneless organelles in health and disease: exploring the molecular basis, physiological roles and pathological implications.Signal Transduct Target Ther. 2024 Nov 18;9(1):305. doi: 10.1038/s41392-024-02013-w. Signal Transduct Target Ther. 2024. PMID: 39551864 Free PMC article. Review.
References
-
- Rubinstein M., Colby R.H. Polymer Physics. Oxford University Press; Oxford, UK: 2003. pp. 137–170.
-
- Huggins M.L. Some properties of solutions of long-chain compounds. J. Phys. Chem. 1942;46:151–158. doi: 10.1021/j150415a018. - DOI
-
- Flory P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942;10:51–61. doi: 10.1063/1.1723621. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

