Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation
- PMID: 36551231
- PMCID: PMC9775968
- DOI: 10.3390/biom12121803
Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation
Abstract
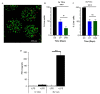
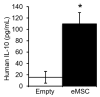
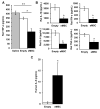
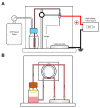
Mesenchymal stem/stromal cells (MSC) promote recovery in a wide range of animal models of injury and disease. They can act in vivo by differentiating and integrating into tissues, secreting factors that promote cell growth and control inflammation, and interacting directly with host effector cells. We focus here on MSC secreted factors by encapsulating the cells in alginate microspheres, which restrict cells from migrating out while allowing diffusion of factors including cytokines across the capsules. One week after intrathecal lumbar injection of human bone marrow MSC encapsulated in alginate (eMSC), rat IL-10 expression was upregulated in distant rat spinal cord injury sites. Detection of human IL-10 protein in rostrally derived cerebrospinal fluid (CSF) indicated distribution of this human MSC-secreted cytokine throughout rat spinal cord CSF. Intraperitoneal (IP) injection of eMSC in a rat model for endotoxemia reduced serum levels of inflammatory cytokines within 5 h. Detection of human IL-6 in sera after injection of human eMSC indicates rapid systemic distribution of this human MSC-secreted cytokine. Despite proof of concept for eMSC in various disorders using animal models, translation of encapsulation technology has not been feasible primarily because methods for scale-up are not available. To scale-up production of eMSC, we developed a rapid, semi-continuous, capsule collection system coupled to an electrosprayer. This system can produce doses of encapsulated cells sufficient for use in clinical translation.
Keywords: alginate; encapsulation; human IL10; mesenchymal stem cells; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sizes and Sufficient Quantities of MSC Microspheres for Intrathecal Injection to Modulate Inflammation in Spinal Cord Injury.Nano Life. 2015 Dec;5(4):1550004. doi: 10.1142/S179398441550004X. Nano Life. 2015. PMID: 29545904 Free PMC article.
-
Alginate encapsulation for bupivacaine delivery and mesenchymal stromal cell immunomodulatory cotherapy.J Inflamm Res. 2019 Mar 12;12:87-97. doi: 10.2147/JIR.S192749. eCollection 2019. J Inflamm Res. 2019. PMID: 30881083 Free PMC article.
-
Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice.J Hepatol. 2015 Mar;62(3):634-41. doi: 10.1016/j.jhep.2014.10.030. Epub 2014 Oct 30. J Hepatol. 2015. PMID: 25450712
-
Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application.Front Cell Dev Biol. 2020 Jul 9;8:497. doi: 10.3389/fcell.2020.00497. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32742977 Free PMC article. Review.
-
Mesenchymal Stem/Stromal Cells Microencapsulation for Cell Therapy.Cells. 2025 Jan 21;14(3):149. doi: 10.3390/cells14030149. Cells. 2025. PMID: 39936941 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cells Loaded in Injectable Alginate Hydrogels Promote Liver Growth and Attenuate Liver Fibrosis in Cirrhotic Rats.Gels. 2025 Mar 27;11(4):250. doi: 10.3390/gels11040250. Gels. 2025. PMID: 40277686 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

