Development of a Vascularized Human Skin Equivalent with Hypodermis for Photoaging Studies
- PMID: 36551256
- PMCID: PMC9775308
- DOI: 10.3390/biom12121828
Development of a Vascularized Human Skin Equivalent with Hypodermis for Photoaging Studies
Abstract
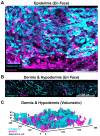
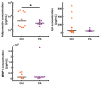
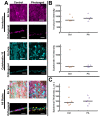
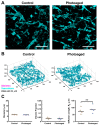
Photoaging is an important extrinsic aging factor leading to altered skin morphology and reduced function. Prior work has revealed a connection between photoaging and loss of subcutaneous fat. Currently, primary models for studying this are in vivo (human samples or animal models) or in vitro models, including human skin equivalents (HSEs). In vivo models are limited by accessibility and cost, while HSEs typically do not include a subcutaneous adipose component. To address this, we developed an "adipose-vascular" HSE (AVHSE) culture method, which includes both hypodermal adipose and vascular cells. Furthermore, we tested AVHSE as a potential model for hypodermal adipose aging via exposure to 0.45 ± 0.15 mW/cm2 385 nm light (UVA). One week of 2 h daily UVA exposure had limited impact on epidermal and vascular components of the AVHSE, but significantly reduced adiposity by approximately 50%. Overall, we have developed a novel method for generating HSE that include vascular and adipose components and demonstrated potential as an aging model using photoaging as an example.
Keywords: HSE; aging; human skin equivalent; photoaging; scaffold; self-assembly; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Farage M.A., Miller K.W., Zouboulis C.C., Piérard G.E., Maibach H.I. Gender differences in skin aging and the changing profile of the sex hormones with age. J. Steroids Horm. Sci. 2012;3:109. doi: 10.4172/2157-7536.1000109. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

