Oxidative Stress-Induced HMGB1 Translocation in Myenteric Neurons Contributes to Neuropathy in Colitis
- PMID: 36551259
- PMCID: PMC9776169
- DOI: 10.3390/biom12121831
Oxidative Stress-Induced HMGB1 Translocation in Myenteric Neurons Contributes to Neuropathy in Colitis
Abstract
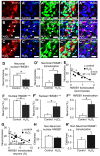
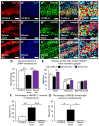
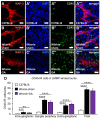
High-mobility group box 1 (HMGB1) is a damage-associated molecular pattern released by dying cells to stimulate the immune response. During cell death, HMGB1 is translocated from the nucleus to the cytoplasm and passively released. High levels of secreted HMGB1 are observed in the faeces of inflammatory bowel disease (IBD) patients, indicating its role in IBD pathophysiology and potential as a non-invasive IBD biomarker. HMGB1 is important in regulating neuronal damage in the central nervous system; its pathological activity is intertwined with oxidative stress and inflammation. In this study, HMGB1 expression in the enteric nervous system and its relevance to intestinal neuroinflammation is explored in organotypic cultures of the myenteric plexus exposed to oxidative stimuli and in Winnie mice with spontaneous chronic colitis. Oxidative stimuli induced cytoplasmic translocation of HMGB1 in myenteric neurons in organotypic preparations. HMGB1 translocation correlated with enteric neuronal loss and oxidative stress in the myenteric ganglia of Winnie mice. Inhibition of HMGB1 by glycyrrhizic acid ameliorated HMGB1 translocation and myenteric neuronal loss in Winnie mice. These data highlight modulation of HMGB1 signalling as a therapeutic strategy to reduce the consequences of enteric neuroinflammation in colitis, warranting the exploration of therapeutics acting on the HMGB1 pathway as an adjunct treatment with current anti-inflammatory agents.
Keywords: HMGB1; colitis; enteric neurons; inflammatory bowel disease; neuroinflammation; neuropathy; oxidative stress; plexitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Bone marrow-derived mesenchymal stem cells mitigate chronic colitis and enteric neuropathy via anti-inflammatory and anti-oxidative mechanisms.Sci Rep. 2024 Mar 20;14(1):6649. doi: 10.1038/s41598-024-57070-6. Sci Rep. 2024. PMID: 38503815 Free PMC article.
-
Inhibition of APE1/Ref-1 Redox Signaling Alleviates Intestinal Dysfunction and Damage to Myenteric Neurons in a Mouse Model of Spontaneous Chronic Colitis.Inflamm Bowel Dis. 2021 Feb 16;27(3):388-406. doi: 10.1093/ibd/izaa161. Inflamm Bowel Dis. 2021. PMID: 32618996 Free PMC article.
-
Mesenchymal stem cell treatment for enteric neuropathy in the Winnie mouse model of spontaneous chronic colitis.Cell Tissue Res. 2022 Jul;389(1):41-70. doi: 10.1007/s00441-022-03633-w. Epub 2022 May 10. Cell Tissue Res. 2022. PMID: 35536444
-
Targeting Enteric Neurons and Plexitis for the Management of Inflammatory Bowel Disease.Curr Drug Targets. 2020;21(14):1428-1439. doi: 10.2174/1389450121666200516173242. Curr Drug Targets. 2020. PMID: 32416686 Review.
-
High Mobility Group Box 1: Biological Functions and Relevance in Oxidative Stress Related Chronic Diseases.Cells. 2022 Mar 1;11(5):849. doi: 10.3390/cells11050849. Cells. 2022. PMID: 35269471 Free PMC article. Review.
Cited by
-
The Oxidative Stress and Nervous Distress Connection in Gastrointestinal Disorders.Biomolecules. 2023 Oct 27;13(11):1586. doi: 10.3390/biom13111586. Biomolecules. 2023. PMID: 38002268 Free PMC article. Review.
-
Advanced Glycation End-Products and Their Effects on Gut Health.Nutrients. 2023 Jan 13;15(2):405. doi: 10.3390/nu15020405. Nutrients. 2023. PMID: 36678276 Free PMC article. Review.
-
Involvement of circadian clock protein PER2 in controlling sleep deprivation induced HMGB1 up-regulation by targeting p300 in the cortex.Sci Rep. 2025 Apr 10;15(1):12253. doi: 10.1038/s41598-025-96931-6. Sci Rep. 2025. PMID: 40210902 Free PMC article.
-
Alterations in tryptophan metabolism and de novo NAD+ biosynthesis within the microbiota-gut-brain axis in chronic intestinal inflammation.Front Med (Lausanne). 2024 Jul 2;11:1379335. doi: 10.3389/fmed.2024.1379335. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39015786 Free PMC article.
-
Bone marrow-derived mesenchymal stem cells mitigate chronic colitis and enteric neuropathy via anti-inflammatory and anti-oxidative mechanisms.Sci Rep. 2024 Mar 20;14(1):6649. doi: 10.1038/s41598-024-57070-6. Sci Rep. 2024. PMID: 38503815 Free PMC article.
References
-
- Gong G., Xiang L., Yuan L., Hu L., Wu W., Cai L., Yin L., Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. - DOI - PMC - PubMed
-
- Stavely R., Fraser S., Sharma S., Rahman A.A., Stojanovska V., Sakkal S., Apostolopoulos V., Bertrand P., Nurgali K. The onset and progression of chronic colitis parallels increased mucosal serotonin release via enterochromaffin cell hyperplasia and downregulation of the serotonin reuptake transporter. Inflamm. Bowel Dis. 2018;24:1021–1034. doi: 10.1093/ibd/izy016. - DOI - PubMed
-
- Filippone R.T., Dargahi N., Eri R., Uranga J.A., Bornstein J.C., Apostolopoulos V., Nurgali K. Potent CCR3 Receptor Antagonist, SB328437, Suppresses Colonic Eosinophil Chemotaxis and Inflammation in the Winnie Murine Model of Spontaneous Chronic Colitis. Int. J. Mol. Sci. 2022;23:7780. doi: 10.3390/ijms23147780. - DOI - PMC - PubMed
-
- Robinson A.M., Rahman A.A., Carbone S.E., Randall-Demllo S., Filippone R., Bornstein J.C., Eri R., Nurgali K. Alterations of colonic function in the Winnie mouse model of spontaneous chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G85–G102. doi: 10.1152/ajpgi.00210.2016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

