Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch
- PMID: 36551265
- PMCID: PMC9775567
- DOI: 10.3390/biom12121837
Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch
Abstract
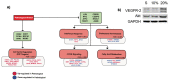
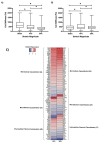
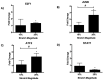
Endothelial cells in vivo are subjected to a wide array of mechanical stimuli, such as cyclic stretch. Notably, a 10% stretch is associated with an atheroprotective endothelial phenotype, while a 20% stretch is associated with an atheroprone endothelial phenotype. Here, a systems biology-based approach is used to present a comprehensive overview of the functional responses and molecular regulatory networks that characterize the transition from an atheroprotective to an atheroprone phenotype in response to cyclic stretch. Using primary human umbilical vein endothelial cells (HUVECs), we determined the role of the equibiaxial cyclic stretch in vitro, with changes to the radius of the magnitudes of 10% and 20%, which are representative of physiological and pathological strain, respectively. Following the transcriptome analysis of next-generation sequencing data, we identified four key endothelial responses to pathological cyclic stretch: cell cycle regulation, inflammatory response, fatty acid metabolism, and mTOR signaling, driven by a regulatory network of eight transcription factors. Our study highlights the dynamic regulation of several key stretch-sensitive endothelial functions relevant to the induction of an atheroprone versus an atheroprotective phenotype and lays the foundation for further investigation into the mechanisms governing vascular pathology. This study has significant implications for the development of treatment modalities for vascular disease.
Keywords: RNA-seq; endothelial cells; mechanotransduction; vascular biology.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

