Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy
- PMID: 36551271
- PMCID: PMC9776383
- DOI: 10.3390/biom12121843
Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy
Abstract
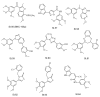
Cancer accounts for numerous deaths each year, and it is one of the most common causes of death worldwide, despite many breakthroughs in the discovery of novel anticancer candidates. Each new year the FDA approves the use of new drugs for cancer treatments. In the last years, the biological targets of anticancer agents have started to be clearer and one of these main targets is tubulin protein; this protein plays an essential role in cell division, as well as in intracellular transportation. The inhibition of microtubule formation by targeting tubulin protein induces cell death by apoptosis. In the last years, numerous novel structures were designed and synthesized to target tubulin, and this can be achieved by inhibiting the polymerization or depolymerization of the microtubules. In this review article, recent novel compounds that have antiproliferation activities against a panel of cancer cell lines that target tubulin are explored in detail. This review article emphasizes the recent developments of tubulin inhibitors, with insights into their antiproliferative and anti-tubulin activities. A full literature review shows that tubulin inhibitors are associated with properties in the inhibition of cancer cell line viability, inducing apoptosis, and good binding interaction with the colchicine binding site of tubulin. Furthermore, some drugs, such as cabazitaxel and fosbretabulin, have been approved by FDA in the last three years as tubulin inhibitors. The design and development of efficient tubulin inhibitors is progressively becoming a credible solution in treating many species of cancers.
Keywords: FDA; cancer; depolymerization; discovery; polymerization; tubulin.
Conflict of interest statement
The author declares no conflict of interest.
Figures
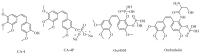
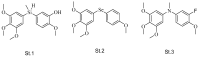

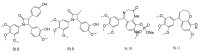
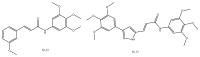

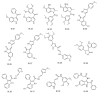
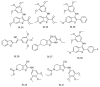
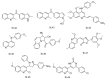
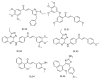
References
-
- Boyle P., Levin B. World Cancer Report 2008. The International Agency for Research on Cancer; Lyon, France: 2008. pp. 12–510. WHO report.
-
- Baytas S.N., Inceler N., Yılmaz A. Synthesis, cytotoxicity, and molecular properties prediction of novel 1,3-diarylpyrazole derivatives. Med. Chem. Res. 2013;22:4893–4908. doi: 10.1007/s00044-013-0505-8. - DOI
-
- Hawash M., Jaradat N., Eid A.M., Abubaker A., Mufleh O., Al-Hroub Q., Sobuh S. Synthesis of novel isoxazole–carboxamide derivatives as promising agents for melanoma and targeted nano-emulgel conjugate for improved cellular permeability. BMC Chem. 2022;16:47. doi: 10.1186/s13065-022-00839-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

