Differential Mobilization of the Phospholipid and Triacylglycerol Pools of Arachidonic Acid in Murine Macrophages
- PMID: 36551279
- PMCID: PMC9775050
- DOI: 10.3390/biom12121851
Differential Mobilization of the Phospholipid and Triacylglycerol Pools of Arachidonic Acid in Murine Macrophages
Abstract
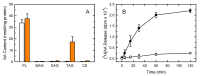
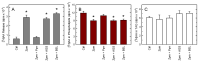
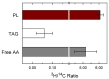
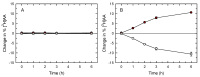
Innate immune cells such as monocytes and macrophages contain high levels of arachidonic acid (AA), part of which can be mobilized during cellular activation for the formation of a vast array of bioactive oxygenated metabolites. Monocytes and macrophages present in inflammatory foci typically incorporate large amounts of AA, not only in membrane phospholipids, but also in neutral lipids such as triacylglycerol. Thus, it was of interest to investigate the metabolic fate of these two AA pools in macrophages. Utilizing a variety of radiolabeling techniques to distinguish the phospholipid and triacylglycerol pools, we show in this paper that during an acute stimulation of the macrophages with yeast-derived zymosan, the membrane phospholipid AA pool acts as the major, if not the only, source of releasable AA. On the contrary, the AA pool in triacylglycerol appears to be used at a later stage, when the zymosan-stimulated response has declined, as a source to replenish the phospholipid pools that were consumed during the activation process. Thus, phospholipids and triacylglycerol play different in roles AA metabolism and dynamics during macrophage activation.
Keywords: arachidonic acid; inflammation; membrane phospholipid; monocytes/macrophages; phospholipase A2; triacylglycerol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Cellular Plasmalogen Content Does Not Influence Arachidonic Acid Levels or Distribution in Macrophages: A Role for Cytosolic Phospholipase A2γ in Phospholipid Remodeling.Cells. 2019 Jul 31;8(8):799. doi: 10.3390/cells8080799. Cells. 2019. PMID: 31370188 Free PMC article.
-
Neutral Lipids Are Not a Source of Arachidonic Acid for Lipid Mediator Signaling in Human Foamy Monocytes.Cells. 2019 Aug 20;8(8):941. doi: 10.3390/cells8080941. Cells. 2019. PMID: 31434356 Free PMC article.
-
Differential roles for triglyceride and phospholipid pools of arachidonic acid in human lung macrophages.J Immunol. 1994 Feb 1;152(3):1394-403. J Immunol. 1994. PMID: 8301140
-
Biochemical functions of a pool of arachidonic acid associated with triglycerides in human inflammatory cells.Int Arch Allergy Immunol. 1995 May-Jun;107(1-3):261-3. doi: 10.1159/000236997. Int Arch Allergy Immunol. 1995. PMID: 7613146 Review.
-
Stimulation of arachidonic acid release and eicosanoid biosynthesis in an interleukin 2-dependent T cell line.J Immunopharmacol. 1986;8(2):165-204. doi: 10.3109/08923978609028614. J Immunopharmacol. 1986. PMID: 3088127 Review.
Cited by
-
Human gut commensal Alistipes timonensis modulates the host lipidome and delivers anti-inflammatory outer membrane vesicles to suppress colitis in an Il10-deficient mouse model.Gut Microbes. 2025 Dec;17(1):2517380. doi: 10.1080/19490976.2025.2517380. Epub 2025 Jun 11. Gut Microbes. 2025. PMID: 40497338 Free PMC article.
-
Integrating uterine microbiome and metabolome to advance the understanding of the uterine environment in dairy cows with metritis.Anim Microbiome. 2024 May 27;6(1):30. doi: 10.1186/s42523-024-00314-7. Anim Microbiome. 2024. PMID: 38802977 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

